Electrochemical methods of analysis- this is a set of methods of qualitative and quantitative analysis based on electrochemical phenomena occurring in the medium under study or at the phase boundary and associated with a change in the structure, chemical composition or concentration of the analyte.
Varieties of the method are electrogravimetric analysis (electroanalysis), internal electrolysis, contact metal exchange (cementation), polarographic analysis, coulometry, etc. In particular, electrogravimetric analysis is based on weighing a substance released on one of the electrodes. The method allows not only to carry out quantitative determinations of copper, nickel, lead, etc., but also to separate mixtures of substances.
In addition, electrochemical methods of analysis include methods based on measuring electrical conductivity (conductometry) or electrode potential (potentiometry). Some electrochemical methods are used to find the end point of a titration (amperometric titration, conductometric titration, potentiometric titration, coulometric titration).
There are direct and indirect electrochemical methods. In direct methods, the dependence of the current strength (potential, etc.) on the concentration of the analyte is used. In indirect methods, the current strength (potential, etc.) is measured in order to find the end point of the titration of the component to be determined with a suitable titrant, i.e. use the dependence of the measured parameter on the volume of the titrant.
For any kind of electrochemical measurements, an electrochemical circuit or an electrochemical cell is required, the component of which is the analyzed solution.
Electrochemical methods are classified depending on the type of phenomena measured during the analysis. There are two groups of electrochemical methods:
1. Methods without superimposing an extraneous potential, based on measuring the potential difference that occurs in an electrochemical cell consisting of an electrode and a vessel with a test solution. This group of methods is called potentiometric. In potentiometric methods, the dependence of the equilibrium potential of the electrodes on the concentration of ions involved in the electrochemical reaction on the electrodes is used.
2. Methods with the imposition of an extraneous potential, based on the measurement of: a) the electrical conductivity of solutions - conductometry; b) the amount of electricity passed through the solution - coulometry; c) the dependence of the current on the applied potential - voltammetry; d) the time required for the passage of an electrochemical reaction - chronoelectrochemical methods(chronovoltammetry, chronoconductometry). In the methods of this group, an extraneous potential is applied to the electrodes of the electrochemical cell.
The main element of instruments for electrochemical analysis is an electrochemical cell. In methods without the imposition of an extraneous potential, it is galvanic cell, in which, due to the occurrence of chemical redox reactions, an electric current arises. In a cell of the type of a galvanic cell, two electrodes are in contact with the analyzed solution - an indicator electrode, the potential of which depends on the concentration of the substance, and an electrode with a constant potential - a reference electrode, relative to which the potential of the indicator electrode is measured. Measurement of the potential difference is carried out with special devices - potentiometers.
In methods with superimposed extraneous potential, electrochemical cell, so named because electrolysis occurs on the electrodes of the cell under the action of an applied potential - the oxidation or reduction of a substance. Conductometric analysis uses a conductometric cell in which the electrical conductivity of a solution is measured. According to the method of application, electrochemical methods can be classified into direct methods, in which the concentration of substances is measured according to the indication of the device, and electrochemical titration, where the indication of the equivalence point is fixed using electrochemical measurements. In accordance with this classification, there are potentiometry and potentiometric titration, conductometry and conductometric titration, etc.
Instruments for electrochemical determinations, in addition to the electrochemical cell, stirrer, load resistance, include devices for measuring the potential difference, current, solution resistance, and the amount of electricity. These measurements can be carried out by pointer instruments (voltmeter or microammeter), oscilloscopes, automatic recording potentiometers. If the electrical signal from the cell is very weak, then it is amplified with the help of radio amplifiers. In devices of methods with superimposed extraneous potential, an important part is the devices for supplying the cell with the appropriate potential of a stabilized direct or alternating current (depending on the type of method). The power supply unit for electrochemical analysis instruments usually includes a rectifier and a voltage stabilizer, which ensures the stability of the instrument.
Potentiometry combines methods based on measuring the emf of reversible electrochemical circuits when the potential of the working electrode is close to the equilibrium value.
Voltammetry is based on the study of the dependence of the polarization current on the voltage applied to the electrochemical cell, when the potential of the working electrode differs significantly from the equilibrium value. It is widely used to determine substances in solutions and melts (for example, polarography, amperometry).
Coulometry combines methods of analysis based on measuring the amount of a substance released at an electrode during an electrochemical reaction in accordance with Faraday's laws. In coulometry, the potential of the working electrode differs from the equilibrium value.
Conductometric analysis is based on a change in the concentration of a substance or the chemical composition of the medium in the interelectrode space; it is not related to the potential of the electrode, which is usually close to the equilibrium value.
Dielectrometry combines methods of analysis based on measuring the dielectric constant of a substance, due to the orientation of particles (molecules, ions) with a dipole moment in an electric field. Dielectrometric titration is used to analyze solutions.
Electrochemical methods of analysis are based on the measurement of potentials, current strength and other characteristics during the interaction of the analyte with an electric current.
Electrochemical methods are divided into three groups:
¨ methods based on electrode reactions occurring in the absence of current (potentiometry);
¨ methods based on electrode reactions occurring under the influence of current (voltammetry, coulometry, electrogravimetry);
¨ methods based on measurements without an electrode reaction (conductometry - low-frequency titration and oscillometry - high-frequency titration).
According to the methods of application, electrochemical methods are classified into straight, based on the direct dependence of the analytical signal on the concentration of the substance, and indirect(establishment of the equivalence point during titration).
To register an analytical signal, two electrodes are required - indicator and comparison. An electrode whose potential depends on the activity of the ions being determined is called indicator. It must quickly and reversibly respond to changes in the concentration of ions to be determined in the solution. An electrode whose potential does not depend on the activity of the ions being determined and remains constant is called reference electrode.
POTENTIOMETRY
Potentiometric method is based on the measurement of the electromotive forces of reversible galvanic cells and is used to determine the concentration of ions in a solution.
The method was developed at the end of the last century, after in 1889 Walter Nernst derived an equation relating the electrode potential to activity (concentration of substances):
where is the standard electrode potential, V; 0.059 is a constant including the universal gas constant (), absolute temperature and Faraday's constant (); is the number of electrons participating in the electrode reaction; and are the activities of the oxidized and reduced forms of the substance, respectively.
When a metal plate is immersed in a solution, an equilibrium is established at the metal-solution interface
Me 0 ↔ Me n+ + nē
and an electrode potential occurs. This potential cannot be measured, but the electromotive force of a galvanic cell can be measured.
The investigated galvanic cell consists of two electrodes, which can be immersed in the same solution (element without transfer) or in two solutions of different composition, having liquid contact with each other (transfer circuit).
An electrode whose potential depends on the activity of the ions being determined is called indicator: E \u003d f (c). An electrode whose potential does not depend on the concentration of the ions being determined and remains constant is called reference electrode. It is used to measure the potential of the indicator electrode.
Electrochemical methods– the most dynamically developing in terms of their application in environmental monitoring. Most often, MOS systems use voltammetry (including polarography), potentiometry (including ionometry), coulometry, and conductometry.
Electrochemical methods of analysis use the dependence of various electrical properties of the medium on the quantitative content and qualitative composition of the substances analyzed in it:
· change capacity electrode depending on the physicochemical processes occurring in the substance ( potentiometric method), incl. selective reactions of ion-selective electrodes, individually sensitive to a large number of cations and anions ( ionometric method);
· change conductivity (current) and permittivity of the substance, depending on the nature of the medium and the concentration of its components ( conductometric and amperometric methods);
changes amount of electricity when the analyte enters the electrochemical cell ( coulometric method);
recovery of the analyzed compound on a mercury dropping or rotating electrode, as a rule, in the analysis of trace amounts of substances in different aggregate states ( polarographic or voltammetric method).
Polarographs of all devices in this group have the highest sensitivity, equal to 0.005–1 µg/ml of sample.
Voltammetry includes a group of electrochemical methods of analysis based on the study of polarization curves. These methods are polarography and amperometric titration - have many varieties and modifications. The most common direct current polarography.
A polarographic setup consists of a direct current source, a voltage divider, a drip (usually mercury) or rotating electrode, and an auxiliary (usually also mercury or other) electrode. To measure the current strength, a microammeter is connected to the system. The electrodes are placed together with the test solution in the electrolyzer (cell).
The voltage applied to the electrolytic cell causes polarization of the anode and cathode E= f a– f k +iR, where i– current strength; TO - solution resistance; f a and f k are the anode and cathode potentials.
If we reduce the resistance of the solution by adding a strong electrolyte (background), then the value iR(potential drop in solution) can be neglected.
The anode potential remains practically constant during the operation of the cell, since the current density is low and the relatively large anode surface is not polarized. Then the potential of a dropping polarizing cathode with a small surface will be equal to: E= -f k. Often in polarographic measurements, instead of a layer of mercury at the bottom of the vessel, a non-polarizable saturated calomel electrode is used, the potential of which is assumed to be zero.
Polarographic data is obtained by measuring the current passing through the electrolytic cell as a function of the potential applied to the electrodes. The graphical dependence of the current strength on the potential is called the polarographic wave ( rice. 2).
At the beginning of electrolysis, with small values of the superimposed EMF, the current strength will be almost constant and increase only very slowly. This is the so-called residual current, which is maintained throughout the electrolysis.
Rice. 2. Polarogram of 10–3 M zinc chloride solution and 1 M potassium chloride solution (curve 1) and 1 M potassium chloride solution (curve 2)
As soon as the ion reduction potential is reached (for example, for the zinc ions being determined, it is equal to -1.0 V), their discharge on a drop of mercury begins:
Zn 2+ + 2 + Hg ® Zn (Hg).
A dilute zinc amalgam Zn (Hg) is formed on the cathode, which decomposes into its constituents as soon as the falling drop comes into contact with the anode:
Zn (Hg) - 2 ® Zn 2+ + Hg.
At the reduction potential of zinc ions, the current increases sharply ( rice. 2), but after reaching a certain value, despite the increase in the applied emf, it remains almost constant. This current is called the limiting or diffusion current, its value is usually proportional to the concentration of the analyte.
When taking polarograms, an indifferent electrolyte with cations that are much more difficult to recover than the analyzed cation is added to the electrolyte under study, for example, KCl, KNO 3, NH 4 Cl; at a concentration 100-1000 times higher than the concentration of the analyte. Such an electrolyte is called "background". It is created in the test solution to increase the electrical conductivity and to shield the electric field of the indicator electrode (cathode). Therefore, the cations of the analyte are not attracted by the electric field of the cathode, but move towards it due to diffusion.
The most important characteristic of a polarogram is the half-wave potential E 1/2 and polarographic wave height h(limiting diffusion current). The half-wave potential is used in quality polarographic analysis. The half-wave potentials of various substances, arranged in ascending order of their negative value, constitute the so-called "polarographic spectrum". Since the half-wave potential significantly depends on the composition of the solution (analyzed medium), the background is always indicated in the polarographic tables.
AT quantitative In polarographic analysis, the methods of calibration curve, additives, comparison and calculation method are used to measure the concentration.
Among the various options for polarography, the method differential pulse polarography (DIP ) is most effective for solving environmental monitoring problems, mainly due to its high sensitivity. The DIP method makes it possible to estimate the content of all substances determined by the classical polarography method. Among other polarographic methods, especially suitable for trace analysis square wave polarography, which provides a detection limit close to the DIP detection limit, but only in the case of reversible electrode processes, and therefore this method is often used to determine traces of heavy metals. The DIP method can also be used to determine surfactants that change the capacitance of the electrical double layer of the electrode.
Methods can be used to determine the microcontents of heavy metal ions. inverse electrochemical analysis (IEA) or in another way, stripping voltammetric analysis (IVA ), in which the metals to be determined are preliminarily deposited on the electrode and then dissolved under polarographic control. This option, in combination with DIP, is one of the most sensitive methods of electrochemical analysis. The hardware design of the IEA (IVA) is relatively simple, which makes it possible to carry out analyzes in the field, and automated continuous control (monitoring) stations can also work on this principle.
IEA (IVA) methods provide the determination of Cu, Pb, Bi, Sb, As, Sn In, Ga, Ag, Tl, Cd, Zn, Hg, Au, Ge, Te, Ni, Co and many anions. An important advantage of IEA (IVA) methods is (unlike other methods, such as atomic absorption spectrometry, for example) the ability to distinguish free ions from their bound chemical forms, which is also important for assessing the physicochemical properties of the analyzed substances from the point of view of ecoanalytical control (for example, when assessing water quality). Many organic substances can also be determined by IEA (IVA) after their adsorption accumulation on the electrode surface.
Polarographic methods can also be used to determine aerosols of various metals in the atmosphere and air of industrial premises after they are captured on appropriate filters, followed by transferring the concentrates into solution. Organic compounds found in the form of gases and vapors in the atmosphere can be determined polarographically after they have been absorbed by specially selected solutions. Metals and various compounds in biological materials are usually determined polarographically after their extraction. All polarographic measurements, including IEA (IVA), can be fully automated, which is essential when performing serial analyses.
One of the most important applications of polarography is the determination of oxygen in water. For this, amperometric detectors are used that generate a current proportional to the oxygen concentration in the solution.
By applying the enzyme to the surface of the detector membrane, it is possible to obtain various enzyme amperometric sensors that are convenient for biochemical and clinical analyzes. Such sensors are also used in environmental monitoring systems.
Electrodes operating on the electrocatalytic principle are suitable for monitoring various gases (SO 2 , H 2 S, CO, NO x) in the air of industrial premises. The electrochemical reactions of these gases (playing the role of a catalyst) occurring on the electrode surface generate a current in the electrode system that is functionally related to the concentration of gases in the air.
The use of polarography is not limited to the analysis of discrete samples, and the method is gradually moving to the principles of continuous analysis of gases and liquids.
Voltammetric polarographic detectors are successfully used in high performance liquid chromatography (HPLC). In this case, the combination of a highly selective separation method with a sensitive detection method leads to a significant expansion of the range of substances determined by the chromatographic method (traces of highly toxic substances, herbicides, drugs, growth stimulants, etc.).
Details of the method can be clarified in the specialized literature ,,,,.
Potentiometry- a method for determining the concentration of substances, based on the measurement of the EMF of reversible galvanic cells.
In practice, two analytical methods are used: direct potentiometry to determine the particle activity, which can be calculated using the Nernst equation from the electromotive force of a galvanic cell, and potentiometric titration , in which a change in the activities of chemicals during the titration process leads to a change in the EMF of a galvanic cell.
The apparatus for carrying out potentiometric titrations and for direct potentiometry is the same. The circuit of potentiometric measurements includes an indicator electrode and a reference electrode with a stable constant potential, as well as a secondary device. The schematic diagram of the method is shown in rice. 3.

1 - indicator electrode; 2 - reference electrode
Rice. 3. Potentiometric cell
The potential of a pair of electrodes is constant. Changing the concentration of the analyte in solution changes the EMF of the circuit. Indicator electrodes usually come in four types, depending on the membrane used, which separates the electrode solution from the test solution: 1) electrodes with a homogeneous membrane of powder or crystalline material; 2) electrodes with a heterogeneous membrane, in which the electrode active substance is distributed, for example, in silicone rubber; 3) electrodes with a liquid membrane, in which the membrane is a solution deposited on a neutral substance, for example, porous glass; 4) glass electrodes with different chemical composition of glass.
Indicator electrodes acquire the potential of the solution in which they are placed. Distinguish two kind indicator electrodes:
1) indifferent electrodes (indestructible during electrolysis);
2) electrodes changing (oxidizing or reducing) during measurements.
Role indifferent electrodes(these are sometimes called electrodes third kind) is to give or add electrons, i.e. be conductors of electricity. Such electrodes can be made of gold, polished platinum, graphite and other materials. Examples of changing electrodes (sometimes referred to as electrodes first kind) can be plates of copper, zinc and other metals, as well as quinhydrone and hydrogen indicator electrodes. The indicator electrodes can also be ion-selective membrane electrodes to determine numerous cations: Li +, Pb +, Cs +, Tl +, NH +, Na +, K +, Ag +, etc. As reference electrodes ( standard electrodes), the potential of which remains constant throughout the measurement, most often used, for example, normal and decinormal calomel (calomel) electrodes with potentials of +0.282 V and +0.334 V, respectively, as well as a saturated silver chloride electrode with a potential of +0.201 V.
In the ideal case, direct potentiometric measurement of the EMF of a galvanic cell can be connected through the Nernst equation with the activity of the particle being determined, or with the concentration, if the corresponding activity coefficients are known:
![]()
where E 0 – standard electrode potential, V; R is the gas constant; T is the absolute temperature; F- Faraday number; n is the number of lost or gained electrons; , [rest.] - equilibrium concentrations of the oxidized, reduced forms, respectively, mol / dm 3.
If we substitute the reference values of the constants and go from the natural logarithm to the decimal logarithm, then for a temperature of 25 ° C we get;

The most important indicator in characterizing the state of the OS is the pH value of this environment, the definition of which ( pH-metry ) is now usually carried out using glass indicator (measuring) electrodes. For long-term measurements, special designs of glass electrodes have been developed with additional devices that clean the glass membrane. Glass electrodes covered with a semi-permeable membrane with an electrolyte film also serve as the basis for various types of probes ( sensors ) used in the analysis of water and air under production conditions for a number of contaminants (NH 3 , CO 2 , NO x , SO 2 , H 2 S, etc.).
The process in the field of creating ion-selective electrodes (ISE) allows you to control the ions F - , I - , Br - , Cl - , CN - , SCN - , NO 3 - , NO 2 - , ClO 4 - , S 2- , Na + , K + Ca 2+ , Ag + , Cu 2+ , Cd 2+ , Pb 2+ in concentration ranges from 10–2 to 10–7 mol/l (approximately 1–10–5 mg/ml). ISE control is characterized by rapidity, simplicity and great possibilities for continuous measurements. ISEs have been developed that are selective to a wide class of organic substances, as well as isomers in their mass, surfactants and detergents in the air of the industrial zone and the water management regime of industrial enterprises.
Potentiometry is also used to measure the redox potentials of various redox (O/W) systems in water. As a rule, the measurement results correspond to a mixed potential, since usually several O/W systems coexist simultaneously in water.
It should be noted that the use of sensors based on semiconductor metal oxide chemically selective and ion-selective field-effect transistors (HSPTs, ISPTs) is promising. Selectivity in these systems is achieved by choosing the composition of the membrane and the layer deposited on the gate of the transistor. The system is immersed in the analyzed solution, and the potential difference between the reference electrode and the gate of the transistor modulates the current flowing between its source and drain. Due to the selectivity of the membrane or the deposited layer, the modulated current becomes a function of the activity of the corresponding component of the solution. Semiconductor sensors form the basis of monitors-analyzers of various gases and vapors. The small size of such sensors makes it possible to combine their aggregates in the form of a mosaic on a single substrate, so that an analyzer is obtained that is capable of monitoring a whole range of harmful substances. Signals from individual sensors included in the mosaic can be sequentially and periodically recorded by the measuring center of the analytical system.
The development of microelectronics makes it possible to design compact probe-type analyzers using modern ISEs. At the same time, a circuit processing the response from the environmental control object, and even a display, can be mounted in the probe handle.
In the special literature, you can familiarize yourself with the details of the method,,,.
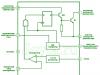
Coulometric the method of analysis is the measurement of the current of the electrode reaction, into which the test substance enters, entering the coulometric cell with the analyzed flow. The schematic diagram of the coulometric cell is shown in rice. four.

1 – cathode chamber; 2 – anode chamber; 3 - microammeter
Rice. four. Diagram of a coulometric cell
Coulometric analysis is based on measuring the amount of electricity used to quantify a given electrochemical process in a given sample, i.e. provided that the current output is 100%. This is the amount of electricity with the help of a current-time integrator connected in series with the measuring cell, or a coulometer-electrolyzer, in which an electrochemical process is carried out with a 100% current output, accompanied by the release of a substance, the amount of which can be easily and accurately restored.
In accordance with Faraday's law:
m( x)/M(x) = m(k)/M(k),
where m(x), m(k) mass of analyte X and the substance released in the coulometer, respectively; M(x), M(k) is the molar mass of substance equivalents X and the substance released in the coulometer, g/mol.

The calculation can also be made according to the equation describing Faraday's law:
![]()
if during the analysis the current strength is measured i, A and time t, s spent on the electrochemical process.
In another modification of this method, called
coulometric titration
, the titrant is generated electrolytically in the analyzed solution at a given current. The consumption of titrant in the analytical reaction is made up for by the charge flowing through the solution during titrant generation until the equivalence point is reached.
One of advantages of coulometric methods is that the titrant standardization process is often not necessary, since the calculations are based on the Faraday constant, i.e. the method is absolute and allows you to estimate the amount of the analyte, and not its concentration. The disadvantage of coulometry with a given potential is the duration of the analysis procedure associated with the need to complete the electrolysis. Computer technology makes it possible to reduce this time by predicting the end of electrolysis by mathematical processing of the current-time curve for the initial stages of electrolysis and by calculating the amount of electricity or the concentration of a substance in a solution. When analyzing multicomponent samples, it can be used scanning coulometry , in which the electrolysis potential is changed continuously or stepwise. For such systems, coulometric titration is preferable to direct coulometry, since 100% current efficiency in titrant generation can be easily achieved by choosing the right titrant reagent and the composition of the working medium. Coulometric titration is applicable to determine from 0.01 to 100 mg of substances (sometimes below 1 μg). The working volume of the samples is usually between 10 and 50 ml. The method is characterized by high accuracy, the relative error does not exceed several tenths of % even with coulometric titration of microgram contents. Under optimal conditions, titration can be performed with a very small total error of 0.01% (rel.). Various acid-base, redox; precipitation and complexometric titration options can be carried out coulometrically.
Coulometric gas analyzers and aqua analyzers (“coulometers”) have been developed and are being produced for the determination of sulfur dioxide and hydrogen sulfide (sulfates and sulfides), ozone (and hydrogen peroxide), chlorine in the air (and active chlorine in water), carbon monoxide and nitrogen dioxide in air (nitrates and nitrites in water). Coulometry is also used as a means of electrochemical detection in liquid chromatography.
Details of the method can be found in the specialized literature.
Conductometric method analysis is based on measuring the electrical conductivity of the solution. The conductometric method of analysis consists in measuring the change in the resistance of an electrolyte solution when a component of the mixture is absorbed. Conductometric installations are used, for example, to determine carbon monoxide and dioxide, gasoline vapors, ammonia and others.
Electrical conductivity is the reciprocal of resistance R, its dimension is CM (Siemens) i.e. æ = 1/ R.
The electrical conductivity of the solution depends on the number of ions per unit volume of the solution, i.e. from concentration FROM, on the mobility of these ions - v. Based on the known relations
![]()
where Z is the distance between the electrodes; S- electrode area; k- coefficient of proportionality.
For a specific pair of electrodes with a constant distance between them S/Z= const. Then
![]() ,
,
where k 1 = k(S/Z).
When calculating in conductometry, the concept of "electrical conductivity" æ 0 is used:
![]()
In calculations, it is convenient to use the equivalent electrical conductivity, which is equal to:
where P - the number of moles of the equivalent in 1 cm 3 of the solution. The equivalent electrical conductivity l ¥ at infinite dilution is equal to the sum of the mobilities of the cation U and anion v.
The ratio of the equivalent electrical conductivity of a weak electrolyte solution to the equivalent electrical conductivity of this electrolyte at infinite dilution is equal to the degree of dissociation a of this electrolyte:
Despite the non-specificity, this method is quite often, compared to other electrochemical methods, used in environmental monitoring systems. This is explained by the fact that when assessing pollution, for example, of water and the atmosphere, not a stage-by-stage, but an output (final) control of industrial processes is possible. Due to the extremely low electrical conductivity of water, it is often enough to estimate the total content of contaminants, which is what conductometry provides. Typical examples of the use of conductometric methods in environmental monitoring are analyzers of detergents in wastewater, concentrations of synthetic components in irrigation systems, quality (salinity) of drinking water. Conductivity analyzers are used for continuous monitoring of air pollution and precipitation, such as SO 2 and H 2 SO 4 . In addition to direct conductometry can be used to identify certain types of pollution indirect methods, which provide very effective estimates of the content of the substances listed above, which interact before measurement with specially selected reagents and the registered change in electrical conductivity is caused only by the presence of the corresponding products in the reaction. So it is possible to determine nitrogen oxides after their catalytic reduction of doammonia, as well as HCl, HBr and CO 2 after a preliminary reaction with Ba(OH) 2 or NaOH. The described principle of CO 2 determination can also be used for indirect determination of organic substances in water.
In addition to classical conductometry, there is also its high-frequency version ( oscillometry ), in which the indicator electrode system is not in contact with the sample. This principle is often implemented in continuous conductometric analyzers.
Electrochemical methods of analysis are also described in a number of educational and special publications,,,,.
LITERATURE
1. Drugov Yu.S., Rodin A.A.Ecological analytical chemistry.
St. Petersburg: 2002. - 464 p.
2. Pashkevich M.A., Shuisky V.F. Environmental monitoring. Tutorial. St. Petersburg State University. - St. Petersburg, 2002. - 90 p.
3. Cattrall Robert W. chemical sensors. M.: Scientific world, 2000. - 144 p.
4. Turyan Ya.I., Ruvinsky O.E., Zaitsev P.M.Polarographic catalimetry. M.: Chemistry, 1998. - 272 p.
5. Budnikov G.K., Maistrenko V.N., Murinov Yu.I. Voltammetry with modified and ultramicroelectrodes. M.: Nauka, 1994. - 239s.
6. Brainina Kh.Z., Neiman E.Ya., Slepushkin V.V. Inversion electroanalytical methods. M.: 1988. - 240 p.
7. Salikhdzhanova R.F. and etc. Polarographs and their operation in practical analysis and research. M.: Chemistry, 1988. - 192 p.
8. Kaplan B.Ya., Pats R.G., Salikhdzhanova R.F. AC voltammetry. M.: Chemistry, 1985. - 264.
9. Bond A.M. Polarographic methods in analytical chemistry. Moscow: Chemistry, 1983.
10. Efremenko O.A. Potentiometric analysis. Moscow: MMA im. THEM. Sechenov, 1998.
11. Reference guide to the use of ion-selective electrodes. M.: Mir, 1986.
12. Korita I. Ions, electrodes, membranes. M.: Mir, 1983.
13. Nikolsky B.V., Materova E.A. ion-selective electrodes. L.: Chemistry, 1980.
14. Efremenko O.A.coulometric titration. Moscow: MMA im. THEM. Sechenov, 1990.
15. Khudyakova T.A., Koreshkov A.P. Conductometric method of analysis. Textbook for universities. M.: Higher school, 1975. - 207 p.
16. Budnikov G.K., Maistrenko V.N., Vyaselev M.R. Fundamentals of modern electroanalysis. Moscow: Chemistry, 2000.
17. Prokhorova G.V. Introduction to electrochemical methods of analysis. M.: Publishing House of Moscow State University, 1991. - 97 p.
18. Electroanalytical Methods in Environmental Control. / Ed. R. Kalvoda, R. Zyka, K. Shtulik et al. M.: Chemistry, 1990. - 240 p.
19. Plambeck J.Electrochemical methods of analysis. Fundamentals of theory and application./ Per. from English. M.: Mir, 1986.
"Electrochemical methods of analysis and their modern hardware design: a review of WEB-sites of firms selling chemical-analytical equipment"
Introduction
Chapter 1. Classification of electrochemical methods
1.1 Voltammetry
1.2 Conductometry
1.3 Potentiometry
1.4 Amperometry
1.5 Coulometry
1.6 Other electrochemical phenomena and methods
1.7 Applied electrochemistry
Chapter 2. Electrochemical methods of analysis and their role in environmental protection
Chapter 3. Devices based on electrochemical methods of analysis
Chapter 4. Review of WEB - sites of firms - sellers of chemical analytical equipment
Literature
INTRODUCTION
Electrochemical methods of analysis (electroanalysis), which are based on electrochemical processes, occupy a worthy place among the methods of monitoring the state of the environment, as they are capable of determining a huge number of both inorganic and organic environmentally hazardous substances. They are characterized by high sensitivity and selectivity, fast response to changes in the composition of the analyzed object, ease of automation and the possibility of remote control. And, finally, they do not require expensive analytical equipment and can be used in laboratory, industrial and field conditions. Three electroanalytical methods are directly related to the problem under consideration: voltammetry, coulometry, and potentiometry.
CHAPTER 1. CLASSIFICATION OF ELECTROCHEMICAL METHODS
Electrochemical methods of analysis (EMA) are based on the study of processes occurring on the electrode surface or in the near-electrode space. The analytical signal is an electrical parameter (potential, current strength, resistance, etc.), functionally related to the concentration of the solution component to be determined and amenable to correct measurement.
The EMA classification proposed by IUPAC has undergone certain changes over the past decades, clarifications (clarifications) and additions have been made to it.
Considerable attention is paid to electrochemical cells and analytical signal sensors (electrode systems, various electrochemical sensors), it is these primary electrochemical converters that determine the analytical capabilities of any method. Currently, the most perfect and fastest processing of the signal from the sensor, the calculation of the statistical characteristics of both the original signal and the results of the entire analysis as a whole is not a problem. That is why it is important to obtain a reliable source signal in order to calibrate it in concentration units.
According to the general classification proposed
IUPAC, EMA are divided into methods in which the excited electrical signal is constant or equal to zero and methods in which the excited signal changes with time. These methods are classified as follows:
voltammetric - voltammetry,I ≠ 0; E = f(t);
potentiometric – potentiometry, (I = 0);
amperometric – amperometry (I ≠ 0; E=const);
chronopotentiometric,E = f(t); I=const;
impedance, or conductometric- measurements using the imposition of an alternating voltage of small amplitude; other, combined(for example, spectroelectrochemical).
1.1 VOLTAMMETRY
VOLTAMMETRY- a set of electrochemical methods of research and analysis based on studying the dependence of the current strength in an electrolytic cell on the potential of an indicator microelectrode immersed in the analyzed solution, on which the investigated electrochemically active (electroactive) substance reacts. In addition to the indicator, an auxiliary electrode with a much larger surface is placed in the cell so that its potential practically does not change when the current passes (non-polarizable electrode). The potential difference between the indicator and auxiliary electrodes E is described by the equation E \u003d U - IR, where U is the polarizing voltage, R is the solution resistance. An indifferent electrolyte (background) is introduced into the analyzed solution in a high concentration in order, firstly, to reduce the value of R and, secondly, to exclude the migration current caused by the action of an electric field on electroactive substances (obsolete - depolarizers). At low concentrations of these substances, the ohmic voltage drop IR in solution is very small. To fully compensate for the ohmic voltage drop, potentiostating and three-electrode cells containing an additional reference electrode are used. In these conditions
As indicator microelectrodes, stationary and rotating ones are used - from metal (mercury, silver, gold, platinum), carbon materials (for example, graphite), as well as dripping electrodes (from mercury, amalgam, gallium). The latter are capillaries from which liquid metal flows drop by drop. Voltammetry using dripping electrodes, the potential of which changes slowly and linearly, called. polarography (the method was proposed by J. Geyrovsky in 1922). Reference electrodes are usually electrodes of the second kind, for example. calomel or silver chloride (see reference electrodes). Dependence curves I =f(E) or I =f(U) (voltammograms) are recorded by special devices - polarographs of various designs.
Voltamperograms obtained using a rotating or dripping electrode with a monotonous change (linear sweep) of the voltage have the form shown schematically in the figure. The section of the increase in current is called. wave. Waves m. b. anodic, if the electroactive substance is oxidized, or cathodic, if it is reduced. When the oxidized (Ox) and reduced (Red) forms of the substance are present in the solution, reacting rather quickly (reversibly) on the microelectrode, the voltammogram shows a continuous cathode-anode wave crossing the abscissa axis at a potential corresponding to the redox potential of the Ox/Red system in this environment. If the electrochemical reaction on the microelectrode is slow (irreversible), the voltammogram shows an anodic wave of oxidation of the reduced form of the substance and a cathodic wave of reduction of the oxidized form (at a more negative potential). The formation of a limiting current area on a voltammogram is associated either with a limited rate of mass transfer of an electroactive substance to the electrode surface by convective diffusion (limiting diffusion current, I d), or with a limited rate of formation of an electroactive substance from the component being determined in solution. Such a current is called the limiting kinetic current, and its strength is proportional to the concentration of this component.
The waveform for a reversible electrochemical reaction is described by the equation:
where R is the gas constant, T is the absolute temperature, E 1/2 is the half-wave potential, i.e. potential corresponding to half the height of the wave (I d /2;). The E 1/2 value is characteristic of a given electroactive substance and is used to identify it. When electrochemical reactions are preceded by the adsorption of the analyte on the electrode surface, the voltammograms show not waves but peaks, which is associated with the extreme dependence of adsorption on the electrode potential. On voltammograms recorded with a linear change (sweep) of the potential with a stationary electrode or on one drop of a dripping electrode (outdated - oscillographic polarogram), peaks are also observed, the descending branch of which is determined by the depletion of the near-electrode layer of the solution with an electroactive substance. The height of the peak is proportional to the concentration of the electroactive substance. In polarography, the limiting diffusion current (in μA) averaged over the lifetime of a drop is described by the Ilkovich equation:
where n is the number of electrons involved in the electrochemical reaction, C is the concentration of the electroactive substance (mM), D is the diffusion coefficient (cm 2 / s), the lifetime of the mercury drop (s), m is the outflow rate of mercury (mg / s) .
With a rotating disk electrode, the limiting diffusion current is calculated by the equation:
where S is the surface area of the electrode (cm 2), is the circular speed of the electrode (rad / s), v is the kinematic viscosity of the solution (cm 2 / s), F is the Faraday number (C / mol).
Cyclic voltammetry (voltammetry with a relatively fast triangular potential sweep) makes it possible to study the kinetics and mechanism of electrode processes by simultaneously observing voltammograms with anodic and cathodic potential sweeps on the screen of an oscilloscope tube with afterglow, reflecting, in particular, the electrochemical reactions of electrolysis products.
The lower limit of the determined concentrations of C n in V. methods with a linear potential sweep is 10 -5 -10 -6 M. To reduce it to 10 -7 -10 -8 M, improved instrumental options are used - alternating current and differential pulse voltammetry.
In the first of these variants, an alternating component of small amplitude of sinusoidal, rectangular (square wave voltammetry), trapezoidal or triangular shape with a frequency usually in the range of 20-225 Hz is imposed on the DC component of the polarization voltage. In the second variant, voltage pulses of the same magnitude (2-100 mV) with a duration of 4-80 ms with a frequency equal to the dropping frequency of a mercury dropping electrode, or with a frequency of 0.3-1.0 Hz when stationary electrodes are used, are applied to the constant component of the polarization voltage. In both cases, the dependence on U or E of the variable current component with phase or time selection is recorded. In this case, voltammograms have the form of the first derivative of a conventional voltammetric wave. The height of the peak on them is proportional to the concentration of the electroactive substance, and the peak potential serves to identify this substance according to reference data.
The peaks of various electroactive substances, as a rule, are better resolved than the corresponding voltammetric waves, and the peak height in the case of an irreversible electrochemical reaction is 5–20 times less than the peak height in the case of a reversible reaction, which also causes an increased resolution of these variants of voltammetry. For example, irreversibly reducing oxygen practically does not interfere with the determination of electroactive substances by alternating current voltammetry. Peaks on alternating current voltammograms reflect not only electrochemical reactions of electroactive substances, but also the processes of adsorption - desorption of non-electroactive substances on the electrode surface (peaks of non-Faraday admittance, obsolete - tensammetric peaks).
For all variants of voltammetry, a method for reducing C n is used, based on preliminary electrochemical, adsorption or chemical accumulation of the solution component to be determined on the surface or in the volume of a stationary microelectrode, followed by registration of a voltammogram reflecting the electrochemical reaction of the accumulation product. This type of voltammetry is called inversion (the outdated name is inversion V. with accumulation on a stationary mercury microelectrode - amalgam polarography with accumulation). In stripping voltammetry with preliminary accumulation, C n reaches 10 -9 -10 -11 M. The minimum values of C n are obtained using thin-film mercury indicator electrodes, incl. mercury-graphite, consisting of tiny droplets of mercury, electrolytically isolated on a substrate of specially treated graphite.
For phase and elemental analysis of solids, stripping voltammetry with electroactive carbon electrodes (the so-called mineral-carbon paste electrodes) is used. They are prepared from a mixture of coal powder, powdered substance under study and an inert binder, for example. vaseline oil. A variant of this method has been developed, which makes it possible to analyze and determine the thickness of metal coatings. In this case, a special device (pressure cell) is used, which makes it possible to record a voltammogram using a drop of background electrolyte deposited on the surface under study.
Application
Voltammetry is used: for the quantitative analysis of inorganic and organic substances in a very wide range of contents - from 10 -10% to tens of%; to study the kinetics and mechanism of electrode processes, including the stage of electron transfer, preceding and subsequent chemical reactions, adsorption of initial products and products of electrochemical reactions, etc.; to study the structure of the electrical double layer c, the equilibrium of complex formation in solution, the formation and dissociation of intermetallic compounds in mercury and on the surface of solid electrodes; to select the conditions of amperometric titration, etc.
1.2 Conductometry
Conductometry - based on measuring the electrical conductivity of a solution and is used to determine the concentration of salts, acids, bases, etc. In conductometric determinations, electrodes of the same materials are usually used, and their conditions are selected in such a way as to minimize the contribution of potential jumps at both electrode/electrolyte interfaces (for example, high-frequency alternating current is used). In this case, the main contribution to the measured cell potential is made by the ohmic voltage drop IR, where R is the resistance of the solution. The electrical conductivity of a one-component solution can be related to its concentration, while measuring the electrical conductivity of electrolytes of complex composition makes it possible to estimate the total content of ions in a solution and is used, for example, in monitoring the quality of distilled or deionized water. In another type of conductometry - conductometric titration - a known reagent is added in portions to the analyzed solution and the change in electrical conductivity is monitored. The equivalence point, at which there is a sharp change in electrical conductivity, is determined from a plot of this value versus the volume of reagent added.
1.3 Potentiometry
Potentiometry - used to determine various physical and chemical parameters based on data on the potential of a galvanic cell. The electrode potential in the absence of current in the electrochemical circuit, measured relative to the reference electrode, is related to the solution concentration by the Nernst equation. In potentiometric measurements, ion-selective electrodes are widely used, which are sensitive mainly to one ion in solution: a glass electrode for measuring pH and electrodes for the selective determination of sodium, ammonium, fluorine, calcium, magnesium, etc. The surface layer of an ion-selective electrode can include enzymes, and the result is a system that is sensitive to the appropriate substrate. Note that the potential of an ion-selective electrode is determined not by the transfer of electrons, as in the case of substances with electronic conductivity, but mainly by the transfer or exchange of ions. However, the Nernst equation, which relates the electrode potential to the logarithm of the concentration (or activity) of a substance in solution, is also applicable to such an electrode. In potentiometric titration, the reagent is added to the analyzed solution in portions and the change in potential is monitored. S-shaped curves, characteristic of this type of titration, allow you to determine the equivalence point and find thermodynamic parameters such as the equilibrium constant and the standard potential.
1.4 Amperometry
The method is based on measuring the limiting diffusion current passing through the solution at a fixed voltage between the indicator electrode and the reference electrode. In amperometric titration, the equivalence point is determined by the break in the curve current - the volume of the added working solution. Chronoamperometric methods are based on measuring the dependence of current on time and are mainly used to determine diffusion coefficients and rate constants. According to the principle of amperometry (as well as voltammetry), miniature electrochemical cells operate, which serve as sensors at the output of liquid chromatograph columns. Galvanostatic methods are similar to amperometric methods, but they measure the potential when a current of a certain value passes through the cell. So, in chronopotentiometry, the change in potential over time is controlled. These methods are mainly used to study the kinetics of electrode reactions.
1.5 Coulometry.
In coulometry at a controlled potential, a complete electrolysis of the solution is carried out, intensively mixing it in an electrolyzer with a relatively large working electrode (bottom mercury or a platinum grid). The total amount of electricity (Q, C) required for electrolysis is related to the amount of the forming substance (A, d) by Faraday's law:
where M is the pier. mass (g/mol), F - Faraday number. Coulometric titration consists in the fact that at direct current a reagent is electrolytically generated that interacts with the substance to be determined. The progress of the titration is controlled potentiometrically or amperometrically. Coulometric methods are convenient in that they are absolute in nature (i.e., they allow you to calculate the amount of the analyte without resorting to calibration curves) and are insensitive to changes in electrolysis conditions and cell parameters (electrode surface area or mixing intensity). In coulombography, the amount of a substance that has undergone electrolysis is determined by weighing the electrode before and after electrolysis.
There are other electroanalytical methods. In alternating current polarography, a sinusoidal voltage of small amplitude is applied to a linearly varying potential over a wide frequency range and either the amplitude and phase shift of the resulting alternating current, or the impedance, is determined. From these data, information is obtained about the nature of substances in solution and about the mechanism and kinetics of electrode reactions. Thin-layer methods use electrochemical cells with an electrolyte layer 10–100 µm thick. In such cells, electrolysis proceeds faster than in conventional electrolyzers. Electrode processes are studied using spectrochemical methods with spectrophotometric registration. To analyze the substances formed on the surface of the electrode, their absorption of light in the visible, UV and IR regions is measured. Changes in the properties of the electrode surface and the medium are monitored using electroreflection and ellipsometry methods, which are based on measuring the reflection of radiation from the electrode surface. These include methods of specular reflection and Raman scattering of light (Raman spectroscopy), second-harmonic spectroscopy (Fourier spectroscopy).
1.6 Other electrochemical phenomena and methods
With the relative motion of the electrolyte and charged particles or surfaces, electrokinetic effects occur. An important example of this kind is electrophoresis, in which the separation of charged particles (for example, protein molecules or colloidal particles) moving in an electric field occurs. Electrophoretic methods are widely used to separate proteins or deoxyribonucleic acids (DNA) in a gel. Electrical phenomena play an important role in the functioning of living organisms: they are responsible for the generation and propagation of nerve impulses, the emergence of transmembrane potentials, and so on. Various electrochemical methods are used to study biological systems and their components. It is also of interest to study the effect of light on electrochemical processes. Thus, the subject of photoelectrochemical research is the generation of electrical energy and the initiation of chemical reactions under the action of light, which is very important for increasing the efficiency of converting solar energy into electrical energy. Semiconductor electrodes made of titanium dioxide, cadmium sulfide, gallium arsenide and silicon are commonly used here. Another interesting phenomenon is electrochemiluminescence, i.e. generation of light in an electrochemical cell. It is observed when high-energy products are formed on the electrodes. Often the process is carried out in a cyclic mode to obtain both oxidized and reduced forms of a given compound. Their interaction with each other leads to the formation of excited molecules, which pass into the ground state with the emission of light.
1.7 Applied electrochemistry
Electrochemistry has many practical applications. With the help of primary galvanic cells (disposable cells) connected to batteries, chemical energy is converted into electrical energy. Secondary current sources - batteries - store electrical energy. Fuel cells are primary power sources that generate electricity by continuously supplying reactants (such as hydrogen and oxygen). These principles underlie portable power sources and batteries used in space stations, electric vehicles and electronic devices.
Large-tonnage production of many substances is based on electrochemical synthesis. During the electrolysis of brine in the chlor-alkali process, chlorine and alkali are formed, which are then used to obtain organic compounds and polymers, as well as in the pulp and paper industry. Electrolysis products are compounds such as sodium chlorate, persulfate, sodium permanganate; industrially important metals are obtained by electroextraction: aluminum, magnesium, lithium, sodium and titanium. It is better to use molten salts as electrolytes, since in this case, unlike aqueous solutions, the reduction of metals is not complicated by hydrogen evolution. Fluorine is obtained by electrolysis in a salt melt. Electrochemical processes serve as the basis for the synthesis of some organic compounds; for example, hydrodimerization of acrylonitrile produces adiponitrile (an intermediate in the synthesis of nylon).
Electroplating of silver, gold, chromium, brass, bronze and other metals and alloys on various objects is widely practiced in order to protect steel products from corrosion, for decorative purposes, for the manufacture of electrical connectors and printed circuit boards in the electronics industry. Electrochemical methods are used for high-precision dimensional processing of workpieces made of metals and alloys, especially those that cannot be processed by conventional mechanical methods, as well as for the manufacture of parts with a complex profile. When anodizing the surface of metals such as aluminum and titanium, protective oxide films are formed. Such films are created on the surface of billets made of aluminum, tantalum and niobium in the manufacture of electrolytic capacitors, and sometimes for decorative purposes.
In addition, investigations of corrosion processes and the selection of materials that slow down these processes are often based on electrochemical methods. Corrosion of metal structures can be prevented using cathodic protection, for which an external source is connected to the protected structure and the anode and the potential of the structure is maintained at such a potential that its oxidation is excluded. Possibilities of practical application of other electrochemical processes are investigated. So, electrolysis can be used to purify water. A very promising direction is the conversion of solar energy using photochemical methods. Electrochemical monitors are being developed, the principle of operation of which is based on electrochemiluminescence.
Electrochemical methods of analysis (electroanalysis), which are based on electrochemical processes, occupy a worthy place among the methods of monitoring the state of the environment, as they are capable of determining a huge number of both inorganic and organic environmentally hazardous substances. They are characterized by high sensitivity and selectivity, fast response to changes in the composition of the analyzed object, ease of automation and the possibility of remote control. And finally, they do not require expensive analytical equipment and can be used in laboratory, industrial and field conditions. Three electroanalytical methods are directly related to the problem under consideration: voltammetry, coulometry, and potentiometry.
Brief historical background. The beginning of the development of electroanalysis is associated with the emergence of the classical electrogravimetric method (circa 1864, W. Gibbs). The discovery by M. Faraday in 1834 of the laws of electrolysis formed the basis of the coulometry method, but the application of this method began in the 30s of the twentieth century. A real turning point in the development of electroanalysis occurred after the discovery in 1922 by J. Heyrovsky of the polarography method. Polarography can be defined as electrolysis with a dropping mercury electrode. This method remains one of the main methods of analytical chemistry. In the late 1950s and early 1960s, the problem of environmental protection stimulated the rapid development of analytical chemistry, and in particular electroanalytical chemistry, including polarography. As a result, improved polarographic methods were developed: alternating current (Barker, B. Breuer) and pulsed polarography (Barksr, A. Gardnsr), which significantly exceeded the classical version of polarography proposed by J. Geyrovsky in their characteristics. When using solid electrodes of various materials instead of mercury (used in polarography), the corresponding methods began to be called voltammetric. At the end of the 1950s, the work of V. Kemuli and Z. Kublik laid the foundation for the stripping voltammetry method. Along with the methods of coulometry and voltammetry, methods are being developed based on measuring the electrode potentials and electromotive forces of galvanic cells - the methods of potentiometry and ionometry (see).
Voltammetry. This is a group of methods based on studying the dependence of the current strength in an electrolytic cell on the magnitude of the potential applied to an indicator microelectrode immersed in the analyzed solution. These methods are based on the principles of electrolysis; the analytes present in the solution are oxidized or reduced at the indicator electrode. In addition to the indicator electrode, a reference electrode with a much larger surface is placed in the cell so that its potential practically does not change when the current passes. As indicator microelectrodes, stationary and rotating electrodes made of platinum or graphite are most often used, as well as a mercury dripping electrode, which is a long narrow capillary, at the end of which small mercury drops 1–2 mm in diameter are periodically formed and detached (Fig. 1). The qualitative and quantitative composition of the solution can be established from voltammograms.
Rice. 4. Electrochemical cell with dropping mercury electrode: 1 - analyzed solution, 2 - dropping mercury electrode, 3 - reservoir with mercury, 4 - reference electrode
Voltammetric methods, especially sensitive variants such as differential pulse polarography and stripping voltammetry, are in constant use in all areas of chemical analysis and are most useful in solving environmental problems. These methods are applicable to the determination of both organic and inorganic substances, for example, to the determination of most chemical elements. With the help of stripping voltammetry, the problem of determining traces of heavy metals in waters and biological materials is most often solved. So, for example, voltammetric methods for the simultaneous determination of Cu, Cd and Pb, as well as Zn and Pb or Ti in drinking water are included in the standard. Germany. An important advantage of voltammetry is the ability to identify the forms of presence of metal ions in waters. This makes it possible to assess the quality of water, since different chemical forms of the existence of metals have different degrees of toxicity. From organic substances, it is possible to determine compounds that have groups capable of reduction (aldehydes, ketones, nitro -, nitroso compounds, unsaturated compounds, halogen-containing compounds, azo compounds) or oxidation (aromatic hydrocarbons, amines, phenols, aliphatic acids, alcohols, sulfur-containing compounds). The possibilities of determining organic substances by stripping voltammetry are significantly expanded when chemically modified electrodes are used. By modifying the electrode surface with polymeric and inorganic films containing reagents with specific functional groups, including biomolecules, it is possible to create conditions for the component to be determined in which the analytical signal will be practically specific. The use of modified electrodes provides selective determination of compounds with similar redox properties (for example, pesticides and their metabolites) or electrochemically inactive on conventional electrodes. Voltammetry is used to analyze solutions, but it can also be used to analyze gases. Many simple voltammetric analyzers have been designed for use in the field.
Coulometry. An analysis method based on measuring the amount of electricity (Q) that has passed through the electrolyzer during the electrochemical oxidation or reduction of a substance at the working electrode. According to Faraday's law, the mass of an electrochemically converted substance (P) is related to Q by the relationship:
P =
QM/
fn,
where M is the molecular or atomic mass of the substance, n is the number of electrons involved in the electrochemical transformation of one molecule (atom) of the substance, p is the Faraday constant.
A distinction is made between direct coulometry and coulometric titration. In the first case, an electrochemically active substance is determined, which is deposited (or transferred to a new oxidation state) on the electrode at a given electrolysis potential, while the amount of electricity consumed is proportional to the amount of the reacted substance. In the second case, an electrochemically active auxiliary reagent is introduced into the analyzed solution, from which a titrant (coulometric titrant) is electrolytically generated, and it quantitatively chemically interacts with the analyte. The content of the component to be determined is estimated by the amount of electricity that passed through the solution during the generation of the titrant until the end of the chemical reaction, which is determined, for example, using color indicators. It is important that, during coulometric analysis, there are no foreign substances in the test solution that can enter into electrochemical or chemical reactions under the same conditions, that is, no side electrochemical and chemical processes occur.
Coulometry is used to determine both trace (at the level of 109-10 R mol/l) and very large amounts of substances with high accuracy. Many inorganic (practically all metals, including heavy metals, halogens, S, NO 3, NO 2) and organic substances (aromatic amines, nitro- and nitroso compounds, phenols, azo dyes) can be determined coulometrically. Automatic coulometric analyzers for the determination of very low concentrations (up to 104%) of gaseous pollutants (SO2 "Oz, H 2 S, NO, N0 2) in the atmosphere have successfully proven themselves in the field.
Potentiometry. An analysis method based on the dependence of the equilibrium electrode potential E on the activity a of the components of the electrochemical reaction: aA + bB + ne = mM + pP.
In potentiometric measurements, a galvanic cell is made up of an indicator electrode, the potential of which depends on the activity of one of the components of the solution, and a reference electrode, and the electromotive force of this element is measured.
There are direct potentiometry and potentiometric titration. Direct potentiometry is used to directly determine the activity of ions by the value of the potential (E) of the corresponding indicator electrode. In the method of potentiometric titration, the change in E is recorded during the reaction of the analyte with a suitable titrant.
When solving problems of environmental protection, the most important method is direct potentiometry using membrane ion-selective electrodes (ISE) - ionometry. Unlike many other methods of analysis, which allow only the total concentration of substances to be estimated, ionometry allows one to estimate the activity of free ions and therefore plays an important role in studying the distribution of ions between their various chemical forms. To control environmental objects, automated monitoring methods are especially important, and the use of ISE is very convenient for this purpose.
One of the main indicators in characterizing the state of the environment is the pH value of the medium, the determination of which is usually carried out using glass electrodes. Glass electrodes coated with a semi-permeable membrane with a film of the corresponding electrolyte are used in the analysis of water and atmosphere to control pollution (NH s, SO 2 NO, NO 2 , CO 2 , H 2 S). ISEs are usually used to control the content of anions, for which there are traditionally much fewer methods of determination than for cations. To date, ISEs have been developed and are widely used for the determination of F, CI, Br, I, C1O 4, CN, S 2, NO] and NO 2, which make it possible to determine the listed ions in the concentration range from 10 -6 to 10 -1 mol / l .
One of the important areas of application of ionometry is hydrochemical research and determination of the concentration of anions and cations in different types of water (surface, sea, rain). Another area of application of ISE is food analysis. An example is the determination of NO - 3 and NO 2 - in vegetables, meat and dairy products, baby food. A miniature ISE in the form of a needle has been created for the determination of NO - 3 directly in the pulp of fruits and vegetables.
Ionometry is also widely used to determine various biologically active compounds and drugs. At present, it can already be said that there are carriers that are selective to almost any type of organic compounds, which means that it is possible to create an unlimited number of corresponding ISEs. A promising direction is the use of enzyme electrodes, the membrane of which includes immobilized enzymes. These electrodes have a high specificity inherent in enzymatic reactions. With their help, for example, it will be possible to determine cholinesterase-inhibiting insecticides (organophosphorus compounds, carbamates) at concentrations of -1 ng/ml. The future of the method is associated with the creation of compact specific sensors, which are modern electronic devices in combination with ion-selective membranes, which will make it possible to dispense with the separation of sample components and significantly speed up analyzes in the field.
Waste water analysis
Electroanalytical methods, which are usually used in the analysis of water to determine inorganic components, are often inferior in sensitivity to the methods of gas and liquid chromatography, atomic absorption spectrometry. However, cheaper equipment is used here, sometimes even in the field. The main electroanalytical methods used in water analysis are voltammetry, potentiometry and conductometry. The most effective voltammetric methods are differential pulsed polarography (DIP) and inversion electrochemical analysis (IEA). The combination of these two methods allows determination with very high sensitivity - approximately 10 -9 mol/l, while the instrumentation is simple, which makes it possible to do analyzes in the field. Fully automated monitoring stations operate on the principle of using the IEA method or a combination of IEA with DIP. The methods of DIP and IEA in the direct version, as well as in combination with each other, are used to analyze water pollution with heavy metal ions and various organic substances. In this case, the methods of sample preparation are often much simpler than in spectrometry or gas chromatography. The advantage of the IEA method is (unlike other methods, for example, atomic absorption spectrometry) also the ability to “distinguish” free ions from their bound chemical forms, which is important both for assessing the physicochemical properties of the analyzed substances and from the point of view of biological control ( for example, when assessing the toxicity of waters). The analysis time is sometimes reduced to a few seconds by increasing the polarizing voltage sweep rate.
Potentiometry using various ion-selective electrodes is used in water analysis to determine a large number of inorganic cations and anions. The concentrations that can be determined in this way are 10 0 -10 -7 mol/l. Control using ion-selective electrodes is characterized by simplicity, rapidity and the possibility of continuous measurements. At present, ion-selective electrodes have been created that are sensitive to certain organic substances (for example, alkaloids), surfactants and detergents. In water analysis, compact probe-type analyzers are used with the use of modern ion-selective electrodes. At the same time, a circuit processing the response and a display are mounted in the probe handle.
Conductometry used in the work of analyzers of detergents in wastewater, in determining the concentration of synthetic fertilizers in irrigation systems, in assessing the quality of drinking water. In addition to direct conductometry, indirect methods can be used to determine certain types of pollutants, in which the substances to be determined interact with specially selected reagents before measurement and the recorded change in electrical conductivity is caused only by the presence of the corresponding reaction products. In addition to the classical versions of conductometry, its high-frequency version (oscillometry) is also used, in which the indicator electrode system is implemented in continuous conductometric analyzers.
Chapter 3. Devices based on electrochemical methods of analysis
The voltammetric method of analysis is today considered one of the most promising among electrochemical methods, due to its wide capabilities and good performance characteristics.
Modern stripping voltammetry, which has replaced classical polarography, is a highly sensitive and rapid method for determining a wide range of inorganic and organic substances with redox properties.
This is one of the most versatile methods for determining trace amounts of substances, which is successfully used to analyze natural geo- and biological, as well as medical, pharmaceutical and other objects.
Voltammetric analyzers make it possible to simultaneously determine several components (up to 4 - 5) in one sample with a fairly high sensitivity of 10 -8 - 10 -2 M (and stripping voltammetry - up to 10 -10 - 10 -9 M).
The most promising in analytical chemistry today is adsorption stripping voltammetry, based on the preliminary adsorption concentration of the element being determined on the electrode surface and subsequent registration of the voltammogram of the resulting product. Thus, it is possible to concentrate many organic substances, as well as metal ions in the form of complexes with organic ligands (especially nitrogen- and sulfur-containing ones). With a sequential accumulation time of 60 s and the use of a differential pulsed voltammogram registration mode, it is possible to achieve detection limits at the level of 10 -10 - 10 -11 mol / l (10 -8 - 10 -9 g / l or 0.01 - 0.001 μg / dm 3 ).
Voltammetric complex for the analysis of metals "IVA - 400MK" (NPKF "Akvilon", Moscow) designed for analysis of 30 elements (Cu, Zn, Pb, Cd, As, Co, Ni, Cr, and other metals), sensitivity 0.1 - 10 -3 µg/dm 3 .
Voltammetric analyzer with UV irradiation of samples - TA-1M (Tomsk), which, in addition to metal ions, allows you to determine a number of organic compounds. The device has the following features:
simultaneous analysis in three electrochemical cells,
a small sample portion (0.1 - 1.0 g),
low cost of sample preparation and analysis.
In St. Pereburg NFT "Volta" produces a voltammetric complex "AVS-1" with a rotating disk glassy carbon electrode, which allows the analysis of toxic elements in water, food products and various materials. The limit of detection without sample concentration is: 0.1 mg/l for Pb, 0.5 mg/l for Cd, 1.0 µg/l for Cu. The sample volume is 20 ml, the time for obtaining the current-voltage curve is no more than 3 minutes.
"AZHE - 12" (Vladikavkaz) is designed for express analysis of the ionic composition of waste and recycled waters. The analyzer uses a traditional mercury electrode. Controlled components - Cu, Zn, Pb, Cd, In, Bi, Tl, Sb, As, Co, Ni, Cr, CN - , Cl - , S 2- . The analyzer allows measurements without sample preparation.
Ecotest-VA (Ekoniks, Moscow)) - portable voltammetric analyzer. It is made on a modern microprocessor element base and is equipped with a whole complex of electrodes - graphite, glassy carbon, precious metal microelectrodes and a mercury dripping electrode.
Devices of this series are designed to determine metals Cu, Zn, Pb, Cd, As, Bi, Mn, Co, Ni, Cr, as well as acetaldehyde, furfural, caprolactam and other substances in samples of drinking, natural, waste water, soil, and after appropriate sample preparation - in food and feed.
The capabilities of many analytical methods for water analysis can be significantly expanded by using flow-injection concentrating attachments operating in automatic mode during sample preparation, for example, of the BPI-M and BPI-N types.
BPI-M - designed for automated sample preparation, it includes microcolumns with highly efficient sorbents. Unit productivity - 30-60 analyzes per day with full automation of the process. The use of the block allows you to increase the sensitivity by 20 times per minute of concentration. The block works best in combination with atomic absorption detection, as well as with X-ray fluorescence, atomic absorption and electrochemical methods.
BPI-N- designed for concentrating metal ions on selective sorbents simultaneously in four microcolumns with DETATA-sorbent or on 4 thin-layer sorption DETATA-filters. It can be used with X-ray fluorescent, atomic absorption, atomic emission, electrochemical methods.
Voltammetric analyzers
Devices based on the principle of inverse voltammetry have recently been in special demand. They combine selectivity and high sensitivity with ease of analysis.
With regard to determining the elemental composition (for example, for heavy metals), these devices successfully compete with atomic absorption spectrophotometers, since they are not inferior to them in sensitivity, but are much more compact and cheaper (about 5–10 times). They do not require additional consumables, and also enable the simultaneous express determination of several elements.
Polarograph ABC - 1.1 (NTF "Volta" St. Petersburg).
The detection limits of metals without sample concentration are (mg/l): Cd, Pb, Bi - 0.0001, Hg - 0.00015, Cu - 0.0005, Zn, Ni - 0.01. The cost is $1700.
Analyzers based on the conductometric principle are designed for the quantitative determination of the total salt content in water. "EKA-2M" (St. Petersburg) measures salinity in a wide range of values from 0.05 to 1000 µS/cm ($900). "ANION", "MARK", KSL (from $330 to $900), COD analyzers ($750).
Gas analyzers of harmful substances
An automatic gas analyzer is a device in which air sampling, determining the amount of a controlled component, issuing and recording analysis results are carried out automatically according to a given program without operator participation. To control the air environment, gas analyzers are used, the operation of which is based on various principles.
Thermal conductometric gas analyzers.
The principle of operation is based on the dependence of the thermal conductivity of the gas mixture on its composition. The sensitive element of analyzers of this type are thin platinum filaments. Depending on the composition of the gas, the temperature of the sensitive element changes, a current arises, the strength of which is proportional to the concentration of the controlled component.
Coulometric gas analyzers.
The principle of operation is based on the measurement of the limiting electric current that occurs during the electrolysis of a solution that contains an analyte, which is an electrochemical depolarizer. The mixture to be analyzed, containing, for example, sulfur dioxide, is fed into the electrochemical cell. It reacts with iodine to form hydrogen sulfide, which is then electrooxidized at the measuring electrode. Electric current is a measure of the concentration of the analyte.
CHAPTER 4 OVERVIEWWEB-SITES OF COMPANIES-SELLERS OF CHEMICAL - ANALYTICAL EQUIPMENT
"AGILENT.RU"
Modern test, measuring and monitoring equipment for the development, manufacture and implementation of new electronic devices and technologies...
http://www.agilent.ru
"ACADEMLINE", CJSC, Moscow
It supplies a wide range of measuring chemical-analytical equipment...
http://www.academline.com/
"AKTAKOM"
The registered trademark AKTAKOM combines a wide range of world-class control and measuring equipment. All the best from foreign and domestic manufacturers...
http://www.aktakom.ru
"ANALITPRIBOR"
Offers gas analyzers
http://www.analytpribor.ru
"WATSON", JSC, Mytishchi, Moscow region
Instruments and measuring instruments;
http://www.watson.ru/
"DIPOLE", NPF, Saint-Petersburg
http://www.dipaul.ru/
"EuroLab SPb", Ltd., St. Petersburg
Spectral analysis instruments, chromatographs.
http://www.eurolab.ru
"IZME.RU"
http://www.izme.ru/
"INSOVT", CJSC
Development and production of gas analyzers
http://www.insovt.ru
"Institute of Information Technologies", Minsk, Belarus
Specializes in the development and production of fiber optics measuring instruments...
"KIPARIS", LLC, St. Petersburg
http://www.kiparis.spb.ru/
"CONTINENT", Gomel
http://www.continent.h1.ru
"Control and measuring instruments and equipment", Volgograd
http://www.oscilloscop.ru
"Kontur", ITC, LLC, Novosibirsk
http://www.kip.ru/
"KraySibStroy", LLC, Krasnoyarsk
http://www.kipkr.ru/
"Chrismas+", CJSC, Saint-Petersburg
http://www.christmas-plus.ru
"KURS", LLC, St. Petersburg
http://www.kypc.spb.ru
"LUMEX", St. Petersburg
http://www.lumex.ru/
"METTEK"
http://www.mettek.ru
"METTLER TOLEDO"
http://www.mt.com
"MONITORING", STC, St. Petersburg
http://www.monitoring.vniim.ru
"Scientific Instruments", OJSC, St. Petersburg
http://www.sinstr.ru
"NevaLab", CJSC, St. Petersburg
http://www.nevalab.ru
"OWEN", PO, Moscow
http://www.owen.ru/
"OKTAVA+", Moscow
http://www.octava.ru/
"OPTEK", CJSC, St. Petersburg
It develops and manufactures gas analyzers and analytical systems for various purposes for use in ecology, industry and scientific research...
http://www.optec.ru
"POLYTECHFORM", Moscow
http://www.ptfm.ru
"Praktik-NC", OJSC, Moscow, Zelenograd
http://www.pnc.ru/
"INSTRUMENTS AND ANALYTICAL EQUIPMENT"
Instruments for chemical analysis.
http://www.zhdanov.ru/
"Sartogosm", CJSC, Saint-Petersburg
http://www.sartogosm.ru
"Special", CJSC, Moscow
http://www.special.ru
"TKA"
http://www.tka.spb.ru/
"TST", CJSC, St. Petersburg
http://www.tst-spb.ru
"ECOPRIBOR", NPO, Moscow
Offers gas analyzers and gas analysis systems...
http://ecopribor.ru
"ECOTECH", SME, Ukraine
http://ecotech.dn.ua
"ECOTECHINVEST", NPF, Moscow
http://ecotechinvest.webzone.ru
"Exis", CJSC, Moscow, Zelenograd
http://www.eksis.ru/
"ELIX"
http://www.eliks.ru/
"EMI", LLC, St. Petersburg
Manufacture of optical gas analyzers, analyzers of oil products.
http://www.igm.spb.ru
"ENERGOTEST", CJSC, Moscow
http://www.energotest.ru, http://www.eneffect.ru
HIMMED
Analytical instruments and chromatography
e-mail:[email protected]
LITERATURE
1. Geyrovsky Ya., Kuta Ya., Fundamentals of polarography, trans. from Czech., M., 1965;
2. Ga l yus Z., Theoretical foundations of electrochemical analysis, trans. from Polish., M., 1974;
3. Kaplan B. Ya., Pulse polarography, Moscow, 1978;
4. Brainina X. 3., Neiman E. Ya., Solid-phase reactions in electroanalytical chemistry, M., 1982;
5. Kaplan B. Ya., Pats R. G., Salikhdzhanova R. M.-F., AC voltammetry, Moscow, 1985.
6. Plambek J. Electrochemical methods of analysis. / Per. from English. M.: Mir, 1985. 496 p.
7. Brief chemical encyclopedia. M.: Soviet Encyclopedia, 1964. Volume 1. A–E. 758 c.
8. Classification and nomenclature of electrochemical methods // Zh. analyte chemistry. 1978. Vol. 33, no. 8. S. 1647–1665.
9. Recommended Terms, Symbols and Definitions for Electroanalytical Chemistry // Pure & Appl. Chem. 1979 Vol. 51. P. 1159–1174.
10. On the use of the concept of "chemical equivalent" and related quantities: Zhurn. analyte chemistry. 1989. Vol. 44, no. 4. S. 762–764; Journal. analyte chemistry. 1982. Vol. 37, no. 5. S. 946; Journal. analyte chemistry. 1982. Vol. 37, no. 5. S. 947.
11. Neiman E.Ya. Terminology of modern analytical chemistry and its formation // Zhurn. analyte chemistry. 1991. Vol. 46, no. 2. S. 393–405.
12. Presentation of the results of chemical analysis (Recommendations IUPAC 1994) // Zh. analyte chemistry. 1998. V. 53. No. 9. S. 999–1008.
13. Compendium of Analytical Nomenclature (Definitive Rules 1997). 3rd ed., IUPAC, Blackwell Science, 1998. 8.1–8.51 (Electrochemical Analysis).
In electrochemical methods for measuring concentration, an electrochemical cell is used. The simplest cell consists of a pair of electrodes immersed in an electrolyte solution. The electrolyte solution is placed in one or two vessels connected by a bridge with electrolyte (transfer cell). The electrodes can be connected directly to each other by a conductor (internal electrolysis) or by conductors through a power source (external electrolysis).
The mechanism of electricity transfer in different parts of the electrical circuit is different. In conductors, electric charge is carried by electrons, in solution - by ions. At the interface, the conduction mechanism changes as a result of a heterogeneous redox reaction. It is called an electrochemical or electrode reaction, that is, a reaction associated with the exchange of charges between chemical compounds that are in different phases - solid (electrode surface) and liquid (electrolyte solution).
There are chemical compounds in solution that easily donate electrons to an electrode made of a certain material, such as platinum or graphite, that is, they oxidize on it. Such an electrode is called an anode. An oxidizing agent is formed on the anode surface, which can remain on it (adsorb), dissolve in the anode material (mercury anode), or diffuse into the electrolyte solution under the action of diffusion forces (concentration gradient).
For example, in a solution of CuCl 2
2Cl - - 2 e= Cl2
(Red 1 - ne= Ox 1)
Solution Pt electrode
Cl - → ←Cl 2
The gaseous Cl 2 formed on the surface of the platinum electrode will diffuse into the electrolyte solution.
There are also chemical compounds in the solution that easily accept electrons from the electrode, i.e. are restored to it. Such an electrode is called a cathode. A reducing agent is formed on the cathode surface, which can remain on it (adsorb), dissolve in the anode material (mercury cathode), or diffuse into the electrolyte solution under the action of diffusion forces.
For example, in a solution of CuCl 2
Cu 2+- + 2e = Cu 0
(Ox 2 + ne = Red 2)
solution Hg-electrode
Cu 2+ → Cu 0 → Cu 0 (Hg)
The copper atoms formed on the surface of the mercury electrode will diffuse deep into the mercury, dissolving in it with the formation of an amalgam.
Both at the anode and at the cathode, new chemical compounds are formed, which were not previously in the solution. If there is a charge transfer from one phase to another, then an electric potential (energy) is established at the interface.
If the electrodes are connected by a conductor, then with a sufficient potential difference between the electrodes, the resistance of the solution to the movement of charges will be overcome and an electric current will flow through the solution (the movement of charges). This current can be measured.
Electrochemical methods of chemical analysis are based on the use of phenomena and processes occurring on the electrode surface, in the near-electrode layer or in the electrolyte solution, associated with the chemical nature and content of components in the solution.
The electrical properties of the electrode-electrolyte system are measured (electrode potential, electric current strength, amount of electricity, electrical conductivity, etc.). All considered electrical quantities depend on the concentration of any components of the electrolyte solution. Therefore, any of them - the electrical conductivity of the electrolyte, the potential of the electrode, the strength of the electric current, the capacitance of the electrical double layer, and others, can serve as an analytical signal if it is functionally related to the concentration of the analyte in the analyzed solution and can be measured. The measured values of the electrical properties are used for quantitative and sometimes qualitative chemical analysis of the composition of a substance.
There are various classifications of electrochemical methods for determining the concentration of a component. For example, methods can be classified as follows.
1. Methods based on the course of the electrode reaction.
1.1. Methods based on the passage of electric current through an electrochemical cell:
-- voltammetry– method, based on measuring the strength of the diffusion current of electrooxidation or electroreduction of the determined component at a certain value of the potential of the indicator electrode;
-- coulometry– method, based on measuring the amount of electricity (Faraday's law) spent on the electrochemical reaction of the component being determined;
-- electrogravimetry– method, based on measuring the mass of the component to be determined, released on the electrode when an electric current passes through the electrolyte solution (Faraday's law);
1.2. Methods based on measuring the potential difference between a pair of electrodes when negligible currents flow in a solution:
-- potentiometry– method, based on the measurement of the potential difference between the indicator electrode and the reference electrode;
2. Methods not related to the course of the electrode reaction:
-- conductometry– method, based on the measurement of the specific electrical conductivity of a solution, which depends on the nature and concentration of the components dissolved in it.
The concentration of the determined component in the sample of the substance of the object of chemical analysis is found, as in any other physical method of chemical analysis, from the calibration graph.
Attention. Instruments for measuring the electrical properties of substances are also used in chemical methods of quantitative chemical analysis, such as titrimetry, in order to fix an equivalent volume of titrant during a chemical reaction. This is the so-called instrumental (indicatorless) way of fixing the equivalence point. Using a means for measuring the electrical properties of substances, the corresponding electrical property of the determined component is measured, which changes with the addition of each portion of the titrant. At the equivalence point, the intensity of the measured property changes sharply and this moment can be fixed by plotting and graphical processing of the titration curve plotted in the coordinates “ measured value of the electrical property – added volume of titrant”. The concentration of the determined component is found from the law of equivalents. This expands the possibilities of titrimetric methods in the analysis of colored, cloudy solutions, aggressive media, etc., where the use of color indicators to fix the equivalence point is impossible. The titration methods in this case are called as follows: potentiometric titration method, conductometric titration method, amperometric titration method, etc. According to the method of comparison with the standard, these methods belong to the chemical methods of quantitative chemical analysis.
The characteristic advantages of electrochemical methods of chemical analysis are the low limit of determination, rapid analysis, ease of measurement with measuring instruments, the possibility of automation and continuity of chemical analysis. However, the processes occurring in electrochemical cells are quite difficult to understand and interpret the results obtained due to their ambiguity, so it is practically impossible to conduct a qualitative analysis of a substance sample with these methods, which limits the possibilities of electrochemical methods for the chemical analysis of substances.
The disadvantage of electrochemical methods of analysis compared to chemical methods of quantitative analysis is their relatively low accuracy (analysis error ~ 10%), however, some methods (coulometry, electrogravimetry) are highly accurate (analysis error ~ 0.01%).