MINISTRY OF HEALTH OF THE RUSSIAN FEDERATION
PHARMACOPEAN ARTICLE
Ginsengthe presentrootsFS.2.5.0013.15
Panacis ginseng radices Instead of GFXI, no. 2, Art. 66
Collected in late August - early September and dried roots of a wild and cultivated perennial herb, ginseng real - Panax ginseng C. A. Mey, this. aralievs - Araliaceae.
AUTHENTICITY
External signs. Whole raw materials. Roots up to 25 cm long, 0.7 - 2.5 cm thick, with 2 - 5 large branches, less often without them. Taproots, longitudinally, less often spiral wrinkled, fragile, even fracture. The "body" of the root is thickened, almost cylindrical, from above with pronounced annular thickenings. In the upper part of the root there is a narrowed transversely wrinkled rhizome - "neck". The rhizome is short with several scars from fallen stems, at the top it forms a "head", which is an expanded remainder of the stem and an apical bud (sometimes 2 - 3). One or more adventitious roots sometimes extend from the "neck". The "neck" and "head" may be missing. The color of the roots from the surface and on the cut is yellowish-white, on a fresh break it is white. The smell is specific. The taste of the aqueous extract is sweet, pungent, then spicy-bitter.
Shredded raw materials. When examining the crushed raw materials under a magnifying glass (10 ×) or a stereomicroscope (16 ×), pieces of roots of various shapes are visible, passing through a sieve with openings of 7 mm. The color from the surface and at the break is yellowish-white. The smell is specific. The taste of the aqueous extract is sweet, pungent, then spicy-bitter.
Powder... When the powder is examined under a magnifying glass (10 ×) or a stereomicroscope (16 ×), a mixture of crushed particles of various shapes of yellowish-white color is seen passing through a sieve with holes of 2 mm. The smell is specific. The taste of the aqueous extract is sweet, pungent, then spicy-bitter.
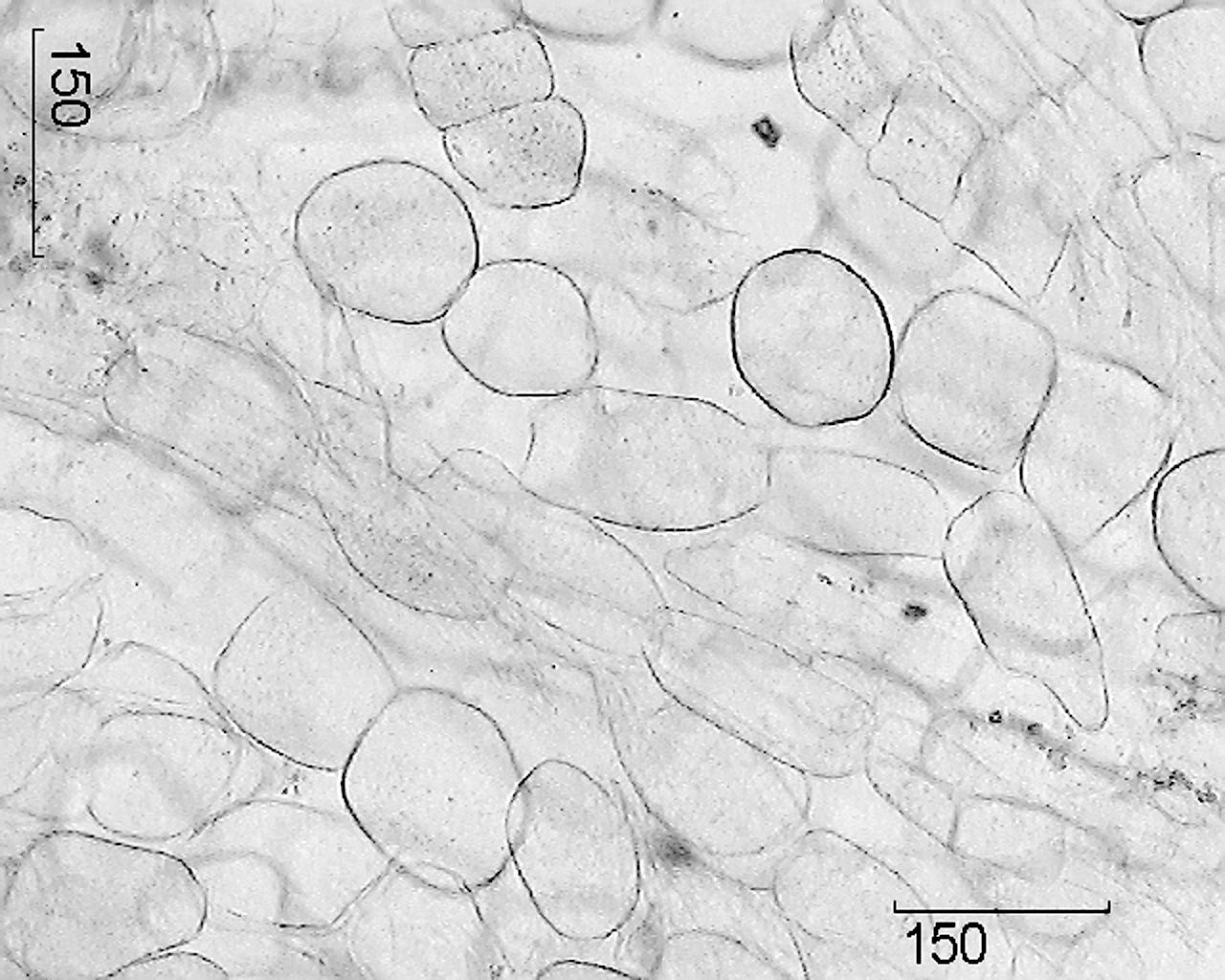
Microscopic signs. Whole raw materials. The cross section of the main root shows a narrow layer of light brown cork, a wide bark, a clear cambium line and wood.
The main root is covered with periderm, the cells of which are thin-walled and lignified, not corked. The phloem and xylem are separated by a cambial zone that passes approximately through the middle of the root radius and
sometimes not visible. To the periphery of the primary xylem, large-cell primary radial rays of the parenchymal tissue depart, between which there is a secondary xylem, crossed by numerous secondary radial rays of the main parenchyma. Xylem consists of thin-walled parenchymal cells containing starch grains. Vessels of the medullary rays have thickened lignified walls and are located singly or collected in groups of 3 - 6. In the parenchyma of wood, cells containing yellow pigments are rarely found. In the center of the root there are indistinctly diagnosed remnants of the primary xylem in the form of 2 rays. The phloem consists mainly of small-celled elements; it contains well-visible schizogenic receptacles containing droplets of secretion from light yellow to red-brown in color. Starch grains are small, round, simple. Druses of calcium oxalate are contained in individual cells of the parenchyma. The outer part of the secondary cortex is bordered by a zone of several (4 - 6) rows of large tangent-elongated parenchymal cells of phelloderm, round or oval, with a slightly thickened membrane.
|
|
||
|
|
|
|
|
|
|
|
Drawing - Ginseng true roots.
1 - a fragment of a cross section of the main root (100 ×); 2 - a fragment of a cork (400 ×); 3 - a fragment of a cross section of the adventitious root: a - xylem vessels, b - starch grains (400 ×); 4 - a fragment of a transverse section of the main root with a secretory canal: a - lining cells of the canal, b - canal cavity (400 ×); 5 - a fragment of the parenchyma of the medullary rays: a - calcium oxalate drusen, b - starch grains (400 ×); 6 - parenchyma cells of the medullary ray (100 ×).
On the cross section of the adventitious root in the center, the ray of the vessels of the primary xylem is the remainder of the diarchic conductive bundle in the primary structure. The two sectors of the secondary xylem are separated by the radial rays of the primary parenchyma. The cells of the parenchyma are round or oval, partially or completely filled with starch grains. The cork consists of 5 - 7 layers of rectangular, thin-walled cells, poorly lignified.
Shredded raw materials... When examining a squashed preparation, fragments of transverse and longitudinal sections of the main and adventitious roots should be visible.
Fragments of the main root are represented by rays and vessels of the xylem, filling the parenchymal cells of the medullary rays with starch grains, canal cavities and lining cells, parenchymal cells with pigments, and cambium cells.
Fragments of the adventitious root are represented by cork cells, parenchyma with starch grains, receptacles, primary and secondary cortex, vessels, medullary rays.
Powder. When examining the micropreparation, fragments of the epidermis, cork, wood, parenchyma, as well as druses of calcium oxalate are visible.
Determination of the main groups of biologically active substances
Thin layer chromatography
On the start line of an analytical chromatographic plate with a layer of silica gel with a fluorescent indicator measuring 10 × 15 cm on an aluminum substrate, apply 20 μl of the test solution (see the section "Quantitative determination" preparation of solution A of the test solution) and 50 μl of a standard sample (SS) solution of panaxoside Rg 1 (see the section "Quantitative determination" preparation of a solution of a CO panaxoside Rg 1). The plate with the applied samples is dried in air, placed in a chamber, pre-saturated for at least 2 hours with a mixture of solvents chloroform - methanol - water (26: 14: 3), and chromatographed in an ascending manner. When the front of solvents passes about 80 - 90% of the length of the plate from the start line, it is removed from the chamber, dried until traces of solvents are removed, treated with phosphotungstic acid with an alcohol solution of 20% and heated in an oven at 100 - 105 ° C for 3 minutes, after which is viewed in daylight.
The chromatogram of the test solution should show at least 6 zones of adsorption from light pink to dark pink; the dominant zone is at the level of the zone on the chromatogram of the CO solution of panaxoside Rg 1; detection of other adsorption zones is allowed.
When a drop of concentrated sulfuric acid is applied to the powder of ginseng roots, after 1 - 2 minutes, a brick-red color appears, turning into red-violet, and then into violet (panaxosides).
Testing
Humidity. Whole raw materials, shredded raw materials, powder - no more than 13%.
Ash is common. Whole raw materials, shredded raw materials, powder - no more than 5%.
Ash insoluble in hydrochloric acid. Whole raw materials, shredded raw materials, powder - no more than 2%.
Grinding of raw materials.Whole raw materials: particles passing through a sieve with openings of 3 mm, no more than 5%. Shredded raw materials: particles that do not pass through a sieve with openings of 7 mm, no more than 5%; particles passing through a sieve with apertures of 0.5 mm, no more than 5%. Powder: particles that do not pass through a sieve with holes of 2 mm, no more than 5%; particles passing through a sieve with holes of 0.18 mm, no more than 5%.
Foreign impurities
Roots darkened from the surface . Whole raw materials, shredded raw materials - no more than 3%.
Organic impurity. Whole raw materials, shredded raw materials - no more than 0.5%.
Mineral admixture . Whole raw materials, shredded raw materials, powder - no more than 1%.
Heavy metals. In accordance with the requirements of the General Pharmacopoeia Monograph "Determination of the content of heavy metals and arsenic in medicinal plant raw materials and medicinal herbal preparations."
Radionuclides. In accordance with the requirements of the General Pharmacopoeia Monograph "Determination of the content of radionuclides in medicinal plant raw materials and medicinal herbal preparations."
Residual amounts of pesticides... In accordance with the requirements of the General Pharmacopoeia Monograph "Determination of the content of residual pesticides in medicinal plant raw materials and medicinal herbal preparations."
Microbiological purity. In accordance with the requirements of the General Pharmacopoeia Monograph "Microbiological Purity".
quantitation. Whole raw materials, shredded raw materials, powder: the amount of panaxosides in terms of panaxoside Rg 1 not less than 2%; extractives extractable with 70% alcohol, not less than 20%.
What is a Pharmacopoeia? If you start from afar, then surely every person at least once had an idea how doctors manage to memorize so many drugs, know their dosages, chemical composition and mechanism of action. In this they are helped by numerous reference books and compendiums containing the necessary information. And their authors, in turn, draw inspiration from the pharmacopoeia. So what is it?
Definition
Pharmacopoeia is a collection of official documents that indicate the quality standards of medicinal raw materials, excipients, finished medicines and other drugs used in medicine.
To establish the "gold standard" specialists in the field of chemistry and pharmaceutical analysis are involved, randomized international double-blind controlled trials are carried out in order to find out everything possible about medicinal raw materials and preparations from them. Compliance with all standards ensures the quality of pharmaceutical products.
The State Pharmacopoeia is a pharmacopoeia that has legal force and is under state supervision. The requirements and recommendations set forth in it are binding on all organizations in the country involved in the manufacture, storage, sale and use of medicines. For violation of the rules fixed in the document, a legal entity or an individual faces criminal liability.
History of the International Pharmacopoeia

The idea of creating a unified list of drugs indicating dosages and standardizing the nomenclature appeared in the scientific medical community at the end of the nineteenth century, in 1874. The first conference on this issue was held in Brussels in 1092. On it, experts came to an agreement on common names for drugs and the form of their prescription in prescriptions. Within four years, this agreement was ratified in twenty countries. This success became the starting point for the further development of the pharmacopoeia and its publication. Twenty years later, the second conference was held in Brussels, which was attended by representatives of forty-one countries of the world.
From that moment on, the responsibility for the publication and revision of the Pharmacopoeia passed to the League of Nations. At the time of the agreement, the guidelines for the preparation and dosage of 77 medicinal substances were included in the compendium. Twelve years later, in 1937, a commission of experts from Belgium, Denmark, France, Switzerland, USA, Netherlands and Great Britain was established, who familiarized themselves with all the provisions of the pharmacopoeia and decided to expand it to an international document.
The Second World War interrupted the work of the commission, but already in 1947 the experts returned to their occupation. By the fifty-ninth year, the commission was called the Expert Committee on the Specification of Pharmaceutical Products. At one of the WHO meetings, it was decided to create a program of International Nonproprietary Names for the unification of the nomenclature of medicines.
First edition

The Pharmacopoeia is an international document that has already had four reprints, and after each of them it acquired something new.
The first edition was approved at the third WHO World Assembly. The permanent secretariat of the International Pharmacopoeia was established. The book was published in 1951, and four years later the second volume was published with additions in three languages common in Europe: English, French and Spanish. After a short period of time, editions appeared in German and Japanese. The first pharmacopoeia is a collection of regulatory documents for all drugs known at that time. Namely:
- 344 articles on medicinal substances;
- 183 articles on dosage forms (tablets, capsules, tinctures, solutions in ampoules);
- 84 methods of laboratory diagnostics.
The headings of the articles were in Latin, since this was the same designation method for all medical workers. To collect the necessary information, experts in biological standardization were involved, as well as narrow specialists on the most endemic and dangerous diseases.
Subsequent editions of the International Pharmacopoeia
The second edition appeared in 1967. It was dedicated to quality control of pharmaceutical products. In addition, errors of the first edition were taken into account and 162 drugs were added.
The third edition of the Pharmacopoeia was targeted at developing countries. It presented a list of substances that are widely used in health care and at the same time have a relatively low cost. This edition contained five volumes and was released in 1975. New edits to the document were made only in 2008. They concerned the standardization of medicines, the methods of their manufacture and distribution.

Pharmacopoeia is a book that combines not only the nomenclature of medicinal substances, but also instructions for their manufacture, storage and purpose. This book contains a description of the chemical, physical and biological methods for the analysis of drugs. In addition, it contains information about reagents and indicators, medicinal substances and preparations.
The WHO Committee compiled lists of poisonous (list A) and potent substances (list B), as well as tables of maximum single and daily doses of drugs.
European Pharmacopoeia

The European Pharmacopoeia is a regulatory document that is used in most European countries in the production of pharmaceutical products along with the International Pharmacopoeia, supplements it and focuses on the peculiarities of medicine in this region. This book was developed by the European Directorate for the Quality of Medicines, which is part of the Council of Europe. The Pharmacopoeia has a different legal status from other similar documents, which was given to it by the Cabinet of Ministers. The official language of the European Pharmacopoeia is French. The last, sixth, reissue was in 2005.
National Pharmacopoeias

Since the International Pharmacopoeia has no legal force and is rather advisory in nature, individual countries have issued national pharmacopoeias for the internal regulation of drug-related issues. At the moment, most countries in the world have individual books. In Russia, the first pharmacopoeia was published in 1778 in Latin. Only twenty years later came the Russian-language version, becoming the first book of this type in the national language.
In 1866, half a century later, the first official Russian-language pharmacopoeia was published. The 11th edition, the last one during the existence of the USSR, appeared in the early nineties of the last century. The preparation, addition and reprinting of the document was previously entrusted to the Pharmacopoeial Committee, but now this is being done by the Ministry of Health, Roszdravnadzor and the General Health Insurance Fund with the involvement of the country's leading scientists.
State Pharmacopoeia of the Russian Federation 12 and 13 editions
In the period of time when the state pharmacopoeia was being adjusted, the quality of medicines was regulated through the pharmacopoeial monographs of the enterprise (FSP) and the general monographs (OFS). The twelfth edition of the State Pharmacopoeia of the Russian Federation was significantly influenced by the fact that Russian specialists were involved in the work of the pharmacopoeia. The twelfth edition consists of five parts, each of which includes basic standards and regulations for the manufacture, prescription or sale of medicines. This book was published in 2009.

Six years later, the twelfth edition was edited. At the end of 2015, the 13th edition of the State Pharmacopoeia appeared on the official website of the Ministry of Health of the Russian Federation. It was an electronic version, since the release was carried out at the expense of funds from sales. Therefore, at the legislative level, it was decided that every pharmacy and wholesaler should have a state pharmacopoeia (13th edition). This made it possible for the book to pay off.
What is a Pharmacopoeia Monograph?
There are two types of substance and finished dosage form. Each article "on substance" has a title in two languages: Russian and Latin, international non-proprietary and chemical name. It contains the empirical and structural formulas, molecular weight and amount of the main active ingredient. In addition, there is a detailed description of the drug substance appearance, quality control criteria, solubility in liquids and other physical and chemical properties. The conditions for packaging, manufacturing, storage and transportation have been agreed. As well as the expiration date.
The article for the finished dosage form, in addition to all of the above, contains the results of clinical and laboratory tests, the permissible deviations for the mass, volume and particle size of the drug substance, as well as the maximum single and daily dosages for children and adults.
Ministry of Health of the Russian Federation
Federal State Budgetary Educational Institution
Higher education
FIRST MOSCOW STATE MEDICAL
UNIVERSITY named after I. M. SECHENOV
PHARMACEUTICAL FACULTY
DEPARTMENT OF PHARMACOGNOSIA
Practical Guide
Pharmacognosy
Topic: Mastering the methods of pharmacognostic analysis
Moscow 2016
TOPIC 1
PHARMACOGNOSTIC ANALYSIS TECHNIQUES
In practical classes, the student receives the skills and practical skills to solve professional problems in the analysis of whole medicinal plant raw materials in accordance with state quality standards.
To implement the competencies in quality control, students should use the State Pharmacopoeia of the Russian Federation (http://www.femb.ru/feml), which reflects modern requirements for the quality of all medicines, including medicinal plant raw materials and medicinal herbal preparations, methods for determining quality and norms ... Federal Law No. 61 "On the Circulation of Medicines" includes Chapter 3 "State Pharmacopoeia".
The Federal State Educational Standard for the specialty "Pharmacy" includes professional competence:
Ø ability and willingness to analyze and assess the quality of medicinal plant materials (used plant organs, histological structure, chemical composition of active and other groups of biologically active substances);
date_______ LESSON 1
DETERMINING THE AUTHENTICITY OF WHOLE LEAVES
Independent work(preparation for the lesson)
Exercise 1. Analyze the OFS. 1.5.1.0001.15 “Medicinal herbal raw materials. Pharmaceutical substances of plant origin ", OFS.1.5.3.0004.15" Determination of the authenticity, grinding and content of impurities in medicinal plant raw materials and herbal medicinal products ", OFS. 1.5.1.0003.15 "Leaves. Folia ”Write down the definitions of the concepts:
« Medicinal plant» -___________________
« Medicinal plant raw materials» - _________
"Pharmaceutical substance of plant origin" -
« Authenticity» - _____________________________
Medicinal plant raw materials " Leaves» - ____
What document regulates the analysis of medicinal plant raw materials "leaves"? ___
Task 2. Sketch the shape of the leaves lily of the valley, stinging nettle, bearberry, woolly foxglove.
Task 3. Draw the venation of the leaves large plantain and large-flowered foxglove.
Task 4. Sketch the edge of the sheet foxglove purple, peppermint, lily of the valley, coltsfoot.
Task 5. Sketch the types of stomatal leaf complexes lingonberry, peppermint, three-leaf watch, belladonna, lily of the valley and give their names.
Task 6. Sketch the types of simple and capitate hairs and give examples of herbal “leaves” where they occur.
| Simple hairs | Capitate hairs | ||||
| Structure | Drawing | LRS | Structure | Drawing | LRS |
| unicellular, smooth | unicellular head on unicellular stalk | ||||
| Unicellular "retort-like" | bicellular head on unicellular stalk | ||||
| 2-4-cell, warty surface | unicellular head on multicellular stalk | ||||
| 3-4-celled, upper cell long, strongly curved | multicellular head with unicellular stalk | ||||
| multicellular head on multicellular stalk |
Write down in which tissue the hairs are located: ________________________________
Task 7. Sketch the types of calcium oxalate inclusions in the leaves stinging nettle, lily of the valley, cassia (senna) holly, belladonna.
Write down in which tissue the calcium oxalate inclusions are located: ____________
Task 8. Sketch the secretory structures found in the leaves. peppermint, wormwood, rod eucalyptus and indicate their location.
"Incoming control has been handed over" ___________________ "____" ________ 20___ G.
(teacher's signature)
WORK IN THE LESSON
Note:
Ø The authenticity of the medicinal plant "leaves" during the lesson is established in accordance with the sections of the FS "External signs" and "Microscopy".
Ø When studying the external signs of leaves, the size and shape (except for leathery leaves) are determined visually on soaked raw materials, the rest of signs - on dry raw materials. The smell is established by grinding the raw materials. The taste is determined only in non-poisonous plants in water extraction or by chewing the raw material (without swallowing).
Ø When microscopic analysis of the sample, it is necessary to establish the localization of diagnostic signs in tissues (epidermis, mesophyll).
Ø Normative documentation is used only at the final stage of the analysis of raw materials to compare the results obtained and write a conclusion on the authenticity of the proposed sample. If the sample of raw materials does not comply with the requirements of the FS, it is necessary to indicate for which sections there is a discrepancy.
Objective 1. Analyze the proposed sample of raw materials in the sections "External signs" and "Microscopy" ND. Draw up the analysis protocol.
ANALYSIS PROTOCOL
Whole medicinal plant raw materials were submitted for analysis (Russian, Latin names)_____
Producing plant (s) ( Russian, Latin names)________________________
The family ( Russian, Latin names)__________
The quality of the analyzed medicinal product is regulated by ( name, number)_____________________
Raw material is _______________________
Exercise 1. Conduct a macroscopic analysis of the raw material and describe its external features in the form of a table:
Task 2. Conduct microscopic analysis of raw materials.
1. Write down the procedure for preparing a micropreparation of a sheet from the surface: _________
2. Prepare a micropreparation of the _________________ sheet from the surface, study it, sketch the anatomical structure and give the designations of the signs.
3. Fill in the table of distribution of diagnostic signs by tissues:
4. Make a conclusion on the compliance of medicinal plant materials with the sections "External signs" and "Microscopy" of the FS.
Conclusion. Raw materials received for analysis ________ ___ meet (do not meet) the requirements of article _____ GF XIII, sections "External signs" and "Microscopy".
Objective 2. Check out the samples of herbarium of medicinal plant raw materials of coltsfoot, plantain, eucalyptus species, medicinal sage, peppermint, lingonberry, bearberry, stinging nettle.
"Lesson minutes passed" ___________________ "____" ________ 20___ G.
(teacher's signature)
Reference materials
State Pharmacopoeia of the Russian Federation, XIII edition, vol. 2
OFS.1.5.1.0001.15 Medicinal herbal raw materials. Pharmaceutical substances
vegetable origin
The requirements of this General Pharmacopoeia Monograph apply to medicinal plant raw materials and pharmaceutical substances of plant origin.
Basic terms and definitions
Medicinal plant raw materials - fresh or dried plants, or their parts, used for the production of medicinal products by drug manufacturing organizations or the manufacture of medicinal products by pharmacy organizations, veterinary pharmacy organizations, individual entrepreneurs licensed to pharmaceutical activity.
Pharmaceutical substance of plant origin - standardized medicinal plant raw materials, as well as substance / substances of plant origin and / or their combinations, products of primary and secondary synthesis of plants, including those obtained from the culture of plant cells, amounts of biologically active substances of plants, products obtained by extraction, distillation, fermentation or other method of processing medicinal plant materials, and used for the prevention and treatment of diseases.
Herbal medicinal product is a medicinal product produced or manufactured from one type of medicinal plant material or several types of such raw material and sold in prepackaged form in secondary (consumer) packaging.
Medicinal plant raw materials can be represented by various morphological groups: grass, leaves, flowers, fruits, seeds, bark, buds, roots, rhizomes, bulbs, tubers, corms and others.
By grinding, medicinal plant raw materials can be:
Whole;
Shredded;
Powder.
Distinguish between medicinal plant materials by the presence of the main groups of biologically active substances used to standardize medicinal plant materials, for example, raw materials containing flavonoids, cardiac glycosides, alkaloids, anthracene derivatives, tannins, etc.
According to the purpose, medicinal plant raw materials are divided into raw materials:
Used for the production of medicinal herbal
preparations (for example, flowers crushed in packs, powder in filter bags);
Used for the manufacture of medicinal herbal
preparations (for example, infusions, decoctions).
PRODUCTION
Medicinal plant raw materials and pharmaceutical substances of plant origin are obtained from cultivated or wild-growing plants. To ensure the quality of medicinal plant raw materials and pharmaceutical substances of plant origin, it is necessary to comply with the relevant rules of cultivation, procurement, drying, grinding and storage conditions. In medicinal plant raw materials and pharmaceutical substances
of plant origin, the content of foreign impurities, both organic (parts of other non-poisonous plants) and mineral (earth, sand, pebbles), is allowed in accordance with the requirements of the General Pharmacopoeia Monograph “Determination of the authenticity, grinding and content of impurities in medicinal plant materials and herbal medicinal products”.
Medicinal plant raw materials and pharmaceutical substances of plant origin used for the production and manufacture of medicinal products must comply with the requirements of the relevant pharmacopoeial monographs or regulatory documents.
To carry out the analysis in order to determine the compliance of the quality of medicinal plant raw materials and pharmaceutical substances of plant origin and medicinal herbal preparations obtained from them with the requirements of the Pharmacopoeia Monograph or regulatory documents, uniform requirements for sampling are established (in accordance with the requirements of the General Pharmacopoeia Monograph “Sampling of medicinal plant materials and medicinal plants preparations ").
In the manufacture of infusions and decoctions from medicinal plant materials and pharmaceutical substances of herbal origin, the water absorption coefficient and the consumption coefficient are determined in accordance with the requirements of the General Pharmacopoeia Monograph “Determination of the water absorption coefficient and consumption coefficient of medicinal plant materials”.
QUALITY INDICATORS AND METHODS OF TESTING MEDICINAL PLANT RAW MATERIALS
Authenticity. Medicinal plant raw materials are identified by macroscopic (external) and microscopic (anatomical) signs (in accordance with the requirements of the General Pharmacopoeia Monograph for the morphological group of raw materials and the General Pharmacopoeia Monograph "Technique of microscopic and microchemical examination of medicinal plants and herbal medicinal products"), and also determine the presence in the analyzed medicinal plant raw materials of the main groups of biologically active substances, confirming its authenticity (in accordance with the requirements of the General Pharmacopoeia Monograph "Determination of the authenticity, grinding and content of impurities in medicinal plant raw materials and herbal preparations"). For this, methods of physicochemical, chemical, histochemical and microchemical analysis are used.
Grinding. The determination is carried out in accordance with the General Pharmacopoeia Monograph "Determination of the authenticity, size and content of impurities in medicinal plant raw materials and herbal medicinal products."
Humidity. The determination is carried out in accordance with the requirements of the General Pharmacopoeia Monograph "Determination of the moisture content of medicinal plants and herbal medicinal products".
Ash is common. The determination is carried out in accordance with the requirements of the General Pharmacopoeia Monograph "Ash total". Does not apply to plant cell culture.
Ash insoluble in hydrochloric acid. The determination is carried out in accordance with the requirements of the General Pharmacopoeia Monograph "Ash insoluble in hydrochloric acid". Does not apply to plant cell culture.
Organic and mineral impurity. The determination is carried out in accordance with the General Pharmacopoeia Monograph "Determination of the authenticity, size and content of impurities in medicinal plant raw materials and herbal medicinal products." Does not apply to plant cell culture.
Pest infestation of stocks. The determination is carried out in accordance with the General Pharmacopoeia Monograph "Determination of the degree of contamination of medicinal plant materials and medicinal plant preparations by pests of stocks". This indicator is assessed in the process of storage of medicinal plant raw materials and when it goes into processing.
Heavy metals. The determination is carried out in accordance with the General Pharmacopoeia Monograph "Determination of the content of heavy metals and arsenic in medicinal plant raw materials and medicinal herbal preparations."
Radionuclides. The determination is carried out in accordance with the General Pharmacopoeia Monograph "Determination of the content of radionuclides in medicinal plant raw materials and medicinal herbal preparations".
Residual amounts of pesticides. The determination is carried out in accordance with the General Pharmacopoeia Monograph "Determination of the content of residual pesticides in medicinal plant raw materials and medicinal herbal preparations" at the stage of the technological process.
Microbiological purity. The determination is carried out in accordance with the General Pharmacopoeia Monograph "Microbiological purity".
Quantitation. The content of biologically active substances that determine the pharmacological effect of medicinal plant materials is determined by the method specified in the monograph or regulatory documentation. The methods used for the quantitative determination of the main groups of biologically active substances should be validated.
Depending on the purpose of medicinal plant raw materials for the same type of medicinal plant raw materials, the norms for the content of one, two or more groups of biologically active substances can be given.
In medicinal plant raw materials, a quantitative determination is carried out:
Extractive substances - in accordance with the requirements of the General Pharmacopoeia Monograph "Determination of the content of extractive substances in medicinal plant raw materials and medicinal herbal preparations";
Essential oil - in accordance with the requirements of the General Pharmacopoeia Monograph "Determination of the content of essential oil in medicinal plant raw materials and medicinal herbal preparations";
Fatty oil - in accordance with the requirements of the General Pharmacopoeia Monograph “Fatty vegetable oils”;
Tannins - in accordance with the requirements of the General Pharmacopoeia Monograph "Determination of the content of tannins in medicinal plant raw materials and herbal medicinal products."
Other groups of biologically active substances in accordance with the requirements of pharmacopoeial monographs or regulatory documents.
The content of biologically active substances related to poisonous and potent substances (cardiac glycosides, alkaloids, etc.) is indicated with the designation of two limits "no less" and "no more". In the case of an overestimated content of these groups of biologically active substances in medicinal plant raw materials, its further use for the production of medicinal products is allowed, which is calculated by the formula:
where t is the amount of medicinal plant materials required for the production of herbal medicinal products, g;
A - the prescribed amount of medicinal plant materials, g:
B - the actual number of units of action in raw materials or the content of biologically active active substances in 1 g of raw materials in%;
B - the standard content of units of action in raw materials or the content of biologically active substances in 1 g of raw materials in%.
Packaging, labeling and transportation. It is carried out in accordance with the requirements of the General Pharmacopoeia Monograph "Packaging, labeling and transportation of medicinal plants and medicinal plants and preparations."
Storage. It is carried out in accordance with the requirements of the General Pharmacopoeia Monograph "Storage of medicinal plants and herbal medicinal products." In the case of using disinfectants, pest control and other agents during the storage of medicinal plant materials, it is necessary to confirm that they do not affect the raw materials and are almost completely removed after processing.
OFS. 1.5.1.0003.15 Leaves. Folia.
Leaves in pharmaceutical practice are called medicinal plant raw materials, which are dried or fresh leaves or individual leaves of a complex leaf. Leaves are usually harvested fully developed, with or without petiole.
External signs. Whole and shredded raw materials. Preparation of objects for analysis:
Small and leathery leaves are examined dry;
Large, thin leaves (usually crumpled) are softened in a humid chamber or by immersion in hot water for a few minutes;
Fresh leaves are examined without pretreatment.
Leaves prepared for analysis are laid out on a glass plate, carefully straightened, examined with the naked eye, using a magnifying glass (10x) or a stereomicroscope (8 *, 16 *, 24 *, etc.). Pay attention to the following anatomical and diagnostic signs:
1. The structure (simple, complex - odd-pinnate, paired-perisgous, double-pinnate, double-pinnate, finger-complex, ternary, etc.) and the size of the leaf blade.
2... Leaf shape(rounded, elliptical, broadly elliptical, narrowly elliptical, oblong, ovate, broadly ovate, narrowly ovate, obovate, orbicularly ovate, broadly ovate, lanceolate, cordate, sagittal, spear-shaped, crescent-shaped, etc.)
3. Depth of dissection of the leaf blade (palmate, pinnate, tricolobate, palmate, pinnate, tripartite, palmate, pinnate, tripartite).
4. The nature of the base (round, broadly, narrowly round, wedge-shaped, narrow-wedge-shaped, broad-wedge-shaped, truncated, notched, heart-shaped, etc.) and the apex (acute, rounded, obtuse, notched, retracted, etc.) of the leaf blade.
5. The nature of the leaf edge (solid, serrate, double-serrate, dentate, crenate, notched).
6. The presence of a petiole, its size.
7. The nature of the petiole surface (smooth, ribbed, grooved, etc.).
8. The presence of the vagina, stipules (free, accrete), characteristics, sizes.
9. Leaf and petiole pubescence (abundance and arrangement of hairs).
10. Leaf vein (in monocots - parallel, arcuate; in dicotyledons - pinnate, finger-like; in ferns and primitive seed plants (gingko) - dichotomous).
11. The presence of essential oil glands and other formations on the surface of the leaf or the presence of receptacles in the mesophyll.
Dimensions are determined using a ruler or graph paper. The length and width of the leaf blade, the length and diameter of the petiole are measured.
The color is determined on both sides of the sheet on dry material in daylight.
The smell is determined by rubbing.
The taste is determined by sampling dry raw materials or aqueous extract of the leaves (only for non-toxic objects).
For crushed leaves, the fineness is determined - the size of the sieve holes through which the mixture of particles passes.
Powder. Examined with the naked eye, using a magnifying glass (10x) or a stereomicroscope (8 *, 16 *, 24 *, etc.). The color of the mixture of particles (total mass and individual inclusions), the shape of the particles, the origin of the particles and their nature (if determined) are noted. When examining under a magnifying glass or stereomicroscope, attention is paid to the pubescence of the fragments, the nature of the surface (smooth, rough, covered with glands, etc.). Determine smell and taste (similar to whole and crushed leaves). Determine the fineness (the size of the sieve holes through which the mixture of particles passes).
Microscopy. Whole and shredded leaves. Prepare micropreparations in accordance with the General Pharmacopoeia Monograph "Technique of microscopic and microchemical examination of medicinal plants and medicinal plant preparations" from whole leaves or pieces of a leaf blade with an edge and a vein, pieces of a leaf from the base and apex, pieces of a petiole (if the leaf has a petiole), examining them from the surface. When analyzing thick and leathery leaves (eucalyptus, bearberry, lingonberry), cross-sections and "crushed" micropreparations are prepared. If necessary, cross sections of the petioles are also prepared.
Pay attention to the following anatomical and diagnostic signs:
1. The nature of the cuticle of the upper and lower epidermis (even; wrinkled, including longitudinally wrinkled, transversely wrinkled, radiantly wrinkled; streaky; comb-like, etc.).
2. The shape of the cells of the upper and lower epidermis (isodiametric - round, square, polygonal; polygonal - rectangular, oval, diamond-shaped, spindle-shaped, combined, etc.); the tortuosity of the cell walls of the upper and lower epidermis (straight, sinuous, wavy, zigzag, jagged, etc.), the degree of tortuosity; thickening of the cell walls of the upper and lower epidermis (uniform, beaded).
3. The presence of stomata, their shape (round, oval), size, frequency of occurrence on the upper and lower epidermis.
4. Type of stomatal apparatus:
Anomocytic type (disordered cell) - anomocytic (or ranunculoid) - the stomata are surrounded by an indefinite number of cells that do not differ in shape and size from other cells of the epidermis;
Diacytic type (two-cell) - the stomata are surrounded by two peri-stomatal cells, the adjacent walls of which are perpendicular to the stomatal gap;
Paracytic type (parallel cell) - on each side of the stomata, along its longitudinal axis, one or more peri-stomatal cells are located;
Anisocytic type (unequal) - the stomata are surrounded by three peri-stomatal cells, one of which is much smaller than the other two;
Tetracytic type - the stomata is surrounded by 4 symmetrically located peri-stomatal cells: two cells are parallel to the stomatal cleft, and the other two are adjacent to the poles of the guard cells;
Hexacite type - the stomata is surrounded by 6 peri-stomatal cells: two pairs are located symmetrically along the guard cells, and two cells occupy polar positions;
Encyclocytic type - side cells form a narrow ring around guard cells;
Actinocyte type - characterized by several side cells, radially diverging from the guard cells.
5. The presence of aquatic stomata (they are large in size and are usually located at the top of the leaf or denticle, above the hydatode).
6. Submergence of stomata into the epidermis (protruding above the epidermis, immersed in the epidermis).
7. The presence and structure of hairs on the upper and lower epidermis (simple and capitate, one- and multicellular, one-, two- and multi-row, bundle, branched and unbranched), their size, features of their attachment points (presence of a rosette), thickened walls (thick, thin walls), the nature of the cuticle (smooth, warty, wry).
8. The presence of glands on the upper and lower epidermis, their structure, size.
9. The presence of secretory canals, lactic acid receptacles (in the parenchyma under the epidermis).
10. The presence and structure of crystalline inclusions (single crystals of various shapes, druses, raffids, styloids, cystolites, crystalline sand, etc.), their localization (in the parenchyma under the epidermis, in the parenchyma in the form of a crystal-bearing sheath around conductive bundles and groups of fibers, rarely in the cells of the epidermis),
11. The presence of inclusions of reserve nutrients: mucus, inulin, etc. (in the parenchyma under the epidermis, less often in the cells of the epidermis).
12. Mesophyll structure (cell shape, uniformity, location, presence of aerenchyma).
13. Leaf structure (dorsoventral, isolateral).
14. The structure of the leaf conducting system (the shape of the main vein; the number, shape, location of the conducting bundles in the vein; the structure of the conducting bundles - the location of the phloem and xylem, the presence of mechanical tissues).
15. The presence of mechanical tissue (collenchyme, sclerenchymal fibers, stony cells, bast fibers, etc.).
16. The structure of the petiole: on the cross section of the leaf petiole, indicate its shape in the middle, basal and apical parts (round, triangular, grooved, sickle-shaped, slightly pterygoid, wide-winged), the number and location of the conducting beams, the presence of mechanical tissue (collenchyma, sclerenchyma).
Powder. Prepare micropreparations of leaf powder in accordance with the General Pharmacopoeia Monograph “Technique of microscopic and microchemical examination of medicinal plant materials and herbal medicinal products”. In micropreparations of the powder, fragments of leaves with the main and secondary veins, fragments of leaves with the edge of the leaf blade, fragments of the leaf apex, fragments in cross-section, fragments of the petiole are examined. In the studied powder particles, all the emerging anatomical and diagnostic signs listed for whole and crushed leaves are noted. Pay attention to the fact that a number of elements (hairs, glands, crystals, druses, etc.) can be separated from the leaf particles; many fragments of tissues and individual elements are observed in the powder: hairs and their fragments, glands, individual crystals of calcium oxalate and fragments of the crystalline sheath, mechanical cells - fibers, sclereids, fragments of secretory canals, receptacles, lactifiers, etc.
In a powder with a particle size of more than 0.5 mm in the fragments under consideration, one can distinguish almost all the features characteristic of whole and crushed raw materials. Some elements of the epidermis can be in the form of fragments of hairs, glands, etc.; due to cell destruction, individual crystals, druses, etc. can occur.
It is even more difficult to isolate anatomical and diagnostic signs in the powder of medicinal plants with a particle size of less than 0.5 mm. There may also be fragments of various parts of the leaf epidermis, however, as much as possible, more attention should be paid to single elements: individual hairs, glands, crystals, cell features, etc.
In the powder of medicinal plants with a particle size of less than 0.5 mm, attention is paid to the structural features of cells and the presence of single elements of the epidermis and mesophyll of the leaf - individual hairs, glands, their fragments, crystals, etc.
The description of the main diagnostic signs should be accompanied by illustrative material.
Luminescence microscopy. Consider a dry powder, less often a cross-section of a sheet, prepared from whole or crushed raw materials after preliminary softening in a humid chamber. The intrinsic (primary) fluorescence of the raw material is observed in ultraviolet light. The cuticle, cell membranes of mechanical tissues, xylem elements, hairs, the contents of individual cells or tissues of the mesophyll, leaf epidermis, depending on their chemical composition, have the brightest glow. The leaves of some plants are characterized by a bright and specific glow of the contents of the glands, secretory canals and receptacles, depending on the chemical composition of the contents.
Qualitative microchemical and histochemical reactions
carried out in micropreparations of leaves (on cross sections, preparations from the surface, in powder), most often in order to detect a thick cuticle, essential oil (can be presented in the form of drops or enclosed in containers and / or tubules), as well as mucus in accordance with the requirements of the General Pharmacopoeia Monograph “Technique of microscopic and microchemical examination of medicinal plants and herbal medicinal products”.
Qualitative reactions are carried out with extraction from the leaves according to the methods given in the monographs or regulatory documents.
Chromatography. Analyze the extracts using various chromatographic techniques using standard samples. Most often, the components of essential oils, flavonoids, etc. are determined chromatographically in extracts from leaves.
Spectrum (UV spectrum). The analysis is carried out in an extract from the leaves if there are appropriate instructions in the monograph or regulatory documentation. Reference to the "Quantification" section is permissible. A description of the conditions for recording the spectrum is given, indicating the wavelengths at which the maximum (s) and minimum (s) absorption should be observed.
In whole, crushed raw materials and powder, determine:
It is possible to determine extractives in accordance with the requirements of the General Pharmacopoeia Monograph "Determination of the content of extractives in medicinal plant raw materials and herbal medicinal products";
Humidity in accordance with the requirements of the General Pharmacopoeia Monograph "Determination of the moisture content of medicinal plants and herbal medicinal products";
hydrochloric acid, in accordance with the requirements of the General Pharmacopoeia Monograph "Ash total" and the General Pharmacopoeia Monograph "Ash insoluble in hydrochloric acid";
Grinding and content of impurities in accordance with the requirements of the General Pharmacopoeia Monograph "Determination of the authenticity, size and
The mass of the contents of the package must comply with the requirements of the General Pharmacopoeia Monograph “Sampling of medicinal plants and herbal medicinal products”.
Pest infestation of stocks. The determination is carried out in accordance with the General Pharmacopoeia Monograph
"Determination of the degree of infestation of medicinal plant materials and medicinal herbal preparations by pests of stocks."
A feature of the current stage of drug standardization is the need to harmonize the requirements for the quality of drugs and methods of their testing, imposed by the Russian Pharmacopoeia and leading foreign pharmacopoeias.
The XII edition of the State Pharmacopoeia of the Russian Federation will include five parts.
The first part describes general provisions, methods of analysis, requirements for pharmaceutical substances, and pharmacopoeial monographs for substances.
The State Pharmacopoeia (GF) is a collection of basic standards used in pharmacopoeial analysis and production of medicines. The State Pharmacopoeia has a legislative character. The State Pharmacopoeia is based on general pharmacopoeial monographs (OFS) and pharmacopoeial monographs (FS). The OFS describes the general provisions, methods of analysis adopted in pharmacopoeial analysis, or includes a list of standardized indicators and test methods for a certain dosage form. The FS determines the level of requirements for specific medicinal products.
CONTENT
I. THE EDITING BOARD OF ROSDRAVNADZOR ON ORGANIZATION OF WORK ON THE STATE PHARMACOPEIA 7
II. FOREWORD 9
III. ORGANIZATIONS, INSTITUTIONS OF RUSSIA AND SPECIALISTS TAKING PART IN THE PREPARATION OF PART 1 OF THE STATE PHARMACOPEIA OF THE RUSSIAN FEDERATION XII EDITION 10
IV. INTRODUCTION 13
GENERAL PHARMACEUTICAL ARTICLES
1. Rules for the use of pharmacopoeial monographs (OFS 42-0031-07) 17
2. Units of the international system (SI) used in the pharmacopoeia and their correspondence to other units (OFS 42-0032-07) 22
ANALYSIS METHODS 26
3. Equipment (OFS42-0033-07) 26
PHYSICAL AND PHYSICOCHEMICAL METHODS OF ANALYSIS 29
4. Melting point (OFS 42-0034-07) 29
5. Solidification temperature (OFS 42-0035-07) 34
6. Temperature limits of distillation and boiling point (OFS 42-0036-07) 36
7. Density (OFS 42-0037-07) 38
8. Viscosity (OFS 42-0038-07) 41
9. Determination of ethyl alcohol in liquid pharmaceutical preparations (OFS 42-0039-07) 49
10. Refractometry (OFS 42-0040-07) 52
11. Polarimetry (OFS 42-0041 -07) 54
12. Spectroscopic methods 56
12.1. Spectrophotometry in the ultraviolet and visible regions (OFS 42-0042-07) 56
12.2. Infrared spectrometry (OFS 42-0043-07) 62
12.3. Atomic emission and atomic absorption spectrometry (OFS 42-0044-07) 66
12.4. Fluorimetry (OFS 42-0045-07) 70
12.5. Nuclear magnetic resonance spectroscopy (OFS42-0046-07) 73
13. Osmolarity (OFS 42-0047-07) 78
14. Ioiom & trt (OFS 42-0048-07) 85
15. Solubility (OFS 42-0049-07) 92
16. Degree of color of liquids (OFS 42-0050-07) 93
17. Transparency and turbidity of liquids (OFS 42-0051-07) 98
CHEMICAL METHODS OF ANALYSIS 101
18. Determination of nitrogen in organic compounds by the Kjeldahl method (OFS 42-0052-0 7) 101
19. Determination of protein (OFS 42-0053-07) 105
20. Nitritometry SOFS 42-0054-0 7) 114
TEST FOR LIMITS OF IMPURITIES 115
21. Total ash (OFS 42-0055-07) 115
22.Sulphated ash (OFS 42-0056-07) 115
23. Residual organic solvents (OFS 42-0057-07) 115
24. Test for Purity and Limits of Impurities 118
24.1. Iron (OFS 42-0058-07) 119
24.2. Heavy metals (OFS 42-0059-07) 121
BIOLOGICAL CONTROL METHODS 124
25. Abnormal toxicity (OFS 42-0060-07) 124
26. Pyrogenicity (OFS 42-0061-07) 125
27. Bacterial endotoxins (OFS 42-0062-07) 128
28. Test for histamine ("OFS 42-0063-07) 136
29. Test for depressants (OFS 42-0064-07) 140
30. Biological methods for assessing the activity of medicinal plants and medicinal products containing cardiac glycosides (OFS 42-0065-07) 141
31. Sterility (OFS 42-0066-0 7) 150
32. Microbiological purity (OFS 42-0067-07) 160
33. Determination of antimicrobial activity of antibiotics by diffusion in agar (OFS 42-0068-0 7) 194
34. Determination of the effectiveness of antimicrobial preservatives of drugs (OFS 42-0069-07) 216
REAGENTS 220
35. Reagents. Indicators (OFS 42-0070-07) 220
36. Titrated solutions (OFS 42-0071-07) 425
37. Buffer solutions (OFS 42-0072-07) 443
38. Radiopharmaceuticals (OFS 42-0073-07) 456
39. Pharmaceutical substances (OFS 42-0074-07) 484
40. Shelf life of medicines (OFS 42-0075-07) 488
Pharmacopoeia articles 493
Free download an e-book in a convenient format, watch and read:
Download the book State Pharmacopoeia of the Russian Federation, XII edition, Part 1, 2007 - fileskachat.com, fast and free download.
Download file No. 1 - doc
Download file number 2 - djvu
Below you can buy this book at the best discounted price with delivery throughout Russia.