MBOU Beyskaya secondary boarding school
secondary (complete) general education
Lecturer - organizer of the life-saving safety Malanchik Pavel Ivanovich.
Outline - outline of a drawing lesson for grade 8
Lesson topic: Drawings of unfolded surfaces of geometric bodies
The purpose of the lesson: Teach you to project an object on 3 planes. Develop spatial thinking. Cultivate accuracy when making drawings.
Methods: Conversation, explanation, demonstration, independent work.
Equipment: Textbook, poster, drawing tools, models.
Lesson type: Learning new material
Lesson structure
Org. moment - 2-3 minutes.
Analysis of graphic work - 5 min.
Fastening - 25 min.
The final part - 3 min.
During the classes
Org. moment.
Hello, sit down.
The topic of today's lesson is "Drawings of unfolded surfaces of geometric bodies." Write it down in a notebook in a drawing font (the topic is written on the board), and at this time I will distribute your work to you.
Setting the goal of the lesson, motivation for the upcoming activity, (it is desirable that the goals of their activities in the lesson are set by the children themselves, two or three people are enough
Analysis of the execution of graphic work.
Put common mistakes on the board, mark the best works.
New material
Drawings of unfolded surfaces of prisms and cylinders.
In the course of the explanation, demonstrate the cut out sweeps, show the sweeps made by children in the past years.
For the manufacture of machine tool fences, ventilation pipes and some other products, their sweeps are cut out of sheet material.
A scan of the surfaces of any straight prism is a flat figure made up of side faces - rectangles and two bases - polygons.
For example, in the sweep of the surfaces of a hexagonal prism (Fig. 139, b), all the faces are equal rectangles of width a and height / i, and the bases are regular hexagons with a side equal to a.
Thus, you can build a drawing of the unfolded surfaces of any prism.
The unfolding of the cylinder surfaces consists of a rectangle and two circles (Fig. 140, b). One side of the rectangle is equal to the height of the cylinder, the other is the circumference of the base. In the drawing of the sweep, two circles are attached to the rectangle, the diameter of which is equal to the diameter of the bases of the cylinder.


Drawings of unfolded surfaces of the cone and pyramid.
The sweep of the surfaces of the cone is a flat figure, consisting of a sector - a sweep of the lateral surface and a circle - the base of the cone (Fig. 141, b).
Constructions are performed like this:
1. Draw a center line and from the point s "on it describe a radius equal to the length s" a "of the generatrix of the cone, an arc of a circle. The circumference of the base of the cone is laid on it.
Point s is connected to the end points of the arc. 2. A circle is attached to the resulting figure - a sector. The diameter of this circle is equal to the diameter of the base of the cone.

The length of a circle when constructing a sector can be determined
according to the formula C = nD.
Angle a is calculated by the formula ![]() ,
,
d - diameter of the base circle,
R is the length of the generator of the cone, it can be calculated by the Pythagorean theorem.
A drawing of a sweep of the surfaces of the pyramid is built as follows
(Fig. 142, b).
An arc of radius R equal to the length of the side edge of the pyramid is described from an arbitrary point O. On this arc, four segments are laid, equal to the side of the base. The extreme points are connected by straight lines to the point O. Then a square is attached equal to the base of the pyramid.
Pay attention to how the drawings of the sweeps are drawn up. Above the image they write "Sweep" with a line below. From the fold lines, which are dash-dotted with two points, draw leader lines and write on the shelf "Fold Lines".

The construction of unfolding is usually performed graphically, using the methods offered by descriptive geometry.
The surfaces of parts bounded by planes or developable curved surfaces can be flattened and aligned with the plane exactly. In this case, points (segments) lying on the surface are preserved on the sweep, and each point (line segment) on the sweep corresponds to a completely definite and unique point (straight line segment) on the surface of the part, and vice versa.
The figure shows unfolded surfaces of polyhedral bodies and bodies of revolution.
The construction of the unfolding of the surface of a polyhedron is reduced to the determination of the natural size of each of its faces. First, a sweep of the lateral surface is drawn, then the bases of the polyhedron are attached to one of the faces (one or two - depending on whether it is a prism or a pyramid

Examples of unfolding polyhedra and bodies of revolution



Anchoring
Together with the children, perform and arrange sweeps of geometric bodies:
Cylinder, Cone, Prism, Pyramid.
In the course of construction, once again dwell on the features of this work. Demonstrate the cut out sweeps, show the sweeps made by children in the past years.
Final part
Summing up.
What did you like in today's lesson?
What did not suit you in this lesson (pace, volume, etc.)?
Have you achieved your goals? Did everyone get the job done?
What have you learned? (it may be worth asking questions here, depending on the time)
Homework: Unroll and glue it. (Any geometric body to choose from, dimensions h - not less than 70mm
The purpose of the assignment- construction of unfolded surfaces with drawing the line of intersection of surfaces.
Given: Drawing "".
Necessary: Construct a cylinder unfolding and mark on it the line of mutual intersection of the surfaces of the cylinder and the hemisphere.
We have already drawn the unfolded cylinder, so we will repeat the material we have studied. Moreover, the original drawing and the method of constructing the original drawing is different from the previous one.
Algorithm for constructing a cylinder unfolding
- We build a scan of the lateral surface of the cylinder.
- Divide the base of the cylinder into 12 equal parts.
- We measure the chord between any two adjacent dividing points of the base circle and plot this distance along the underside of the cylinder sweep.
- We attach the base of the cylinder to any generatrix of the lateral surface.
- We draw the line of intersection of the cone and the cylinder on the sweep of the lateral surface of the cylinder.
Since we have only one projection (frontal) of the mutual intersection of the cylinder and the hemisphere, we will construct only the profile projection of the cylinder. The profile projection of the cylinder with all the auxiliary constructions necessary for constructing the unfolding of the cylinder will be selected with thin lines and will be considered auxiliary constructions.
For more details, see the video tutorial.
Video "Unfolding a cylinder"
This video tutorial and article are included in the professional free AutoCAD tutorial, which is suitable for both novice users and those who have already been working in AutoCAD for a long time.
Abstract of a drawing lesson.
Theme: Drawings of sweeps of some geometric bodies.
Goals:
- to consolidate the concept of geometric bodies;
Contribute to the independent study of the construction of sweeps of geometric bodies;
Develop spatial representations and thinking, the ability to work with information sources;
To foster a sense of time, responsibility in the team.
Lesson type: a lesson in learning new material
Material support: models of geometric bodies, cards - assignments, textbooks, drawing accessories, drawing paper.
DURING THE CLASSES:
1. Organizational part.
Very correct, very wise
May laziness not be a hindrance
In the morning, say to everyone: "Good ... (morning)",
Well, in the afternoon say: "Good .. (day)."
View student readiness for a lesson.
Are you ready to start the lesson!
Is everything in place? Is everything all right:
Books, pens, pencils and notebooks?
We have a motto like this:
Everything you need at hand!
2. Updating knowledge
In the previous lessons, we examined some geometric bodies, learned how to build their drawings. Let's remember what geometric bodies are there?
I show, and the students call.
Let's check how you learned the material you learned.
What is the order of the projections?(frontal, horizontal and profile).
One of them works at the blackboard (Yura), making projections of the cone, and the rest work independently in their notebooks.
The height of the cone is L = 40 mm, and the base diameter is 30 mm.
3. Learning new material.
Lesson topic message.
Today we will continue working with geometric bodies, the topic of today's lesson: “ Drawings of sweeps of some geometrical bodies ".
In the lesson, we have to learn on our own how to unfold some geometric bodies.
We often meet with sweeps of surfaces in everyday life, in production, in construction. To make packaging for juice, sweets, perfumes, a holiday box or bag, etc., you need to be able to build a sweep of the surfaces of geometric bodies.
Consider the unfolding of the packages and tell me what geometric shapes do they consist of?
What is a sweep? Let's open the tutorials on page 63 and read the definition.
Now I will show you how to unfold some geometric bodies.
Unfold the surface of the pyramid.
In order to unroll, let's define what shapes the pyramid consists of.
The side surface of the pyramid consists of four equal triangles. To build a triangle, you need to know the size of its sides. Equal edges of the pyramid serve as lateral sides of the faces (triangles). From an arbitrary point, we describe an arc with a radius equal to the length of the side edge of the pyramid. On this arc, lay four segments equal to the side of the base. We connect the extreme points with straight lines to the center of the described arc. Then we add a square equal to the base of the pyramid.
Development of cylinder surfaces.
The unfolding of the lateral surface of the cylinder consists of a rectangle and two circles. One side of the rectangle is equal to the height of the cylinder, the other is the circumference of the base.
The circumference is calculated by the formula: L = Pi * D.
In the drawing of the sweep, two circles are attached to the rectangle, the diameter of which is equal to the diameter of the base of the cylinder.
When drawing up drawings of sweeps, a sign is applied over the image of the figure -
Fold lines should be drawn with a dash-and-dot line with two dots.
All clear? To consolidate the new material, we will perform practical work in pairs on the cards. And one at the board will unfold the cube.

4. Practical work in pairs. Before starting work, please tell me with what tools and with what material you will work?
5. Summing up.
What new did you learn in the lesson?
What did you meet?
Where are they used?
What have you learned?
6. Reflection.
Did you enjoy the lesson?
Are you satisfied with your work in the lesson?
You have emoticons on your desk.
Choose the emoticon that matches the grade of your work in the lesson.
7. Assessment of students.
I am grateful to you for the lesson, for the fact that you worked well. I hope that your interest in learning drawing will not fade away.
Goodbye!
Task card. Development of the cylinder (page 65. fig. 137).
Height H = 40mm, D = 40mm.

Task card. Unfold pyramid (page 64. fig. 134).
50mm, A = 40mm.
Task card. Sweep of a triangular prism (page 65. fig. 136).
Prism height H = 40mm, base side A = 30mm

Task card. Unfolding the cube (page 64. fig. 132).
Side of the cube A = 30mm.

Introduction date 1974-07-01
This standard establishes the basic requirements for the implementation of drawings of parts, assembly, overall and assembly at the stage of development of working documentation for all industries.
(Changed edition, Amendment No. 8,).
1.GENERAL REQUIREMENTS FOR WORKING DRAWINGS
1.1. General Provisions
1.1.1. When developing working drawings, they provide for:
a) optimal use of standard and purchased products, as well as products mastered by production and corresponding to the state of the art;
b) a rationally limited range of threads, splines and other structural elements, their sizes, coatings, etc .;
c) a rationally limited range of grades and assortments of materials, as well as the use of the cheapest and least scarce materials;
d) the necessary degree of interchangeability, the most advantageous methods of manufacturing and repairing products, as well as their maximum ease of maintenance in operation.
1.1.1a. Working drawings on paper (in paper form) and electronic drawings can be made on the basis of an electronic model of a part and an electronic model of an assembly unit ( GOST 2.052).
General requirements for electronic documents - according to GOST 2.051
1.1.2. For references in the drawings of serial and mass production products for technical conditions, the latter must be registered in the prescribed manner (in states where state registration of technical conditions is mandatory).
It is allowed to give references to technological instructions when the requirements established by these instructions are the only ones that guarantee the required quality of the product; at the same time, they must be attached to the set of design documentation for the product when transferred to another enterprise.
It is not allowed to give references to documents defining the shape and dimensions of structural elements of products (chamfers, grooves, etc.), if there is no symbolic designation of these elements in the relevant standards. All data for their manufacture should be shown in the drawings.
(Changed edition, Amendments No. 4, 10,).
1.1.3. It is not allowed to place technological instructions on working drawings. As an exception, it is allowed:
a) indicate the methods of manufacture and control, if they are the only ones that guarantee the required quality of the product, for example, joint processing, joint bending or flaring, etc.;
b) give instructions on the choice of the type of technological workpiece (castings, forgings, etc.);
c) indicate a certain technological method that guarantees the provision of individual technical requirements for the product, which cannot be expressed by objective indicators or quantities, for example, the aging process, vacuum impregnation, gluing technology, control, coupling of a plunger pair, etc.
1.1.4. For products of the main unit * and auxiliary production on the drawings intended for use at a specific enterprise, it is allowed to place various instructions on the manufacturing technology and product control.
* The rules for the execution of drawings of products of one-off production also apply to auxiliary production.
1.1.6. The dimensions of the conventional signs not established in the standards are determined taking into account the clarity and clarity of the drawing and are kept the same with repeated repetition.
1.1.7. On the working drawing of the product indicate the dimensions, limit deviations, surface roughness and other data to which it must correspond before assembly (Fig. a).
An exception is the case specified in cl.
The dimensions, maximum deviations and roughness of the surfaces of the elements of the product resulting from processing during the assembly process or after it are indicated on the assembly drawing (Fig. b).
1.1.14. If an edge (edge) must be made sharp or rounded, then a corresponding instruction is placed on the drawing. If there are no indications on the shape of the edges or ribs in the drawing, then they should be blunt.
If necessary, in this case, you can specify the size of the blunting (chamfer, radius), placed next to the "∟" sign, for example dash. ...
(Modified edition, Amendment No. 9).
1.2.6. In the drawing of the product obtained by cutting the workpiece into parts and interchangeable with any other product made from other workpieces to the submitted drawing, the image of the workpiece is not placed (Fig.).
1.2.7. For a product obtained by cutting a workpiece into parts or consisting of two or more jointly processed parts, used only in conjunction and not interchangeable with the same parts of another of the same product, one drawing is developed (Fig.).
1.3. Product drawings with additional processing or alteration
1.3.1. Drawings of products manufactured with additional processing of other products are performed taking into account the following requirements:
a) the blank product is depicted by solid thin lines, the apsurfaces obtained by additional processing, newly introduced products and products installed instead of the existing ones - by solid main lines.
The parts removed during the alteration are not depicted;
b) apply only those dimensions, maximum deviations and designations of surface roughness that are necessary for additional processing (drawing).
It is allowed to apply reference, overall and connection dimensions. It is allowed to depict only a part of the blank product, the elements of which must be additionally processed.
1.3.2. In the drawing of a part manufactured by additional processing of the workpiece, in the column 3 the main inscription write down the word " Workpiece»And designation of the blank product.
When using a purchased product as a blank product, in column 3 of the main inscription indicate the name of the purchased product and its designation, which are contained in the accompanying documentation of the manufacturer (supplier).
(Modified edition, Amendment No. 11)
Assembly drawing

Drawings
The positions of the components included in the options will interfere with the corresponding additional images (Fig.).
3.3.14. In cases where individual parts of the purchased product are installed in different assembly units of the product (for example, tapered roller bearings), the purchased product is recorded in the specification of the assembly unit in which it is assembled. In the technical requirements of the assembly drawing of the product being developed, those assembly units are indicated, which include individual parts of the purchased product. In the specifications of these assembly units in the "Note" column indicate the designation of the specification, which includes the purchased product in assembled form. In this case, in the column "Name" indicate the name of the component part of the purchased product, and the column "Quantity." is not filled.
(Added additionally, Amendment No. 8).
4 DIMENSIONAL DRAWINGS
4.1. Dimensional drawings are not intended for the manufacture of products for them and should not contain data for manufacture and assembly.
4.2. In the dimensional drawing, the image of the product is performed with maximum simplifications. The product is depicted so that the extreme positions of moving, extending or folding parts, levers, carriages, hinged covers, etc. are visible.
It is allowed not to show elements that protrude beyond the main contour by an insignificant amount in comparison with the dimensions of the product.
4.3. The number of views in the dimensional drawing should be minimal, but sufficient to give a comprehensive idea of the external outlines of the product, the positions of its protruding parts (levers, flywheels, handles, buttons, etc.), the elements that should be constantly in the field of view (for example, scales), on the location of the elements of connection of the product with other products.
4.4. The image of the product in the dimensional drawing is performed with solid main lines, and the outlines of the moving parts in the extreme positions are dash-dotted thin lines with two dots.
It is allowed to depict the extreme positions of the moving parts in separate views.
(Modified edition, Amendment No. 3).
4.5. In the dimensional drawing, it is allowed to depict parts and assembly units that are not part of the product as continuous thin lines.
4.6. On the dimensional drawing, the overall dimensions of the product, the installation and connection dimensions and, if necessary, the dimensions that determine the position of the protruding parts are applied.
Installation and connection dimensions required for linking with other products must be indicated with maximum deviations. It is allowed to indicate the coordinates of the center of mass. The dimensional drawing does not indicate that all dimensions shown on it are for reference only.
(Modified edition, Amendment No. 8).
4.7. The dimensional drawing is allowed to indicate the conditions of use, storage, transportation and operation of the product in the absence of these data in the technical description, technical specifications or other design documents for the product.
4.8. An example of a dimensional drawing is shown in fig. ...
5.8. The products and materials required for installation, which are not supplied by the assembled product, are recorded in the list on the installation drawing, and in the “Note” column or in the technical requirements, a corresponding note is placed, for example: “Pos. 7 and 9 not supplied with the product ", etc.
If it is impossible to indicate the exact designations and names of the products not supplied, then their approximate names are indicated in the list, and in the drawing, if necessary, dimensions and other data that ensure the correct selection of products required for installation.
5.9. On the installation drawing, on the shelf of the leader line or directly on the image, indicate the name and (or) designation of the device (object) or part of the device to which the mounted product is attached.
INFORMATION DATA
1. DEVELOPED AND INTRODUCEDState Committee of Standards of the Council of Ministers of the USSR
2. APPROVED AND INTRODUCED INACTION By the Decree of the State Committee of Standards of the Council of Ministers of the USSR dated July 27, 1973 No. 1843
Amendment No. 9 was adopted by the Interstate Council for Standardization, Metrology and Certification (Minutes No. 13 of May 28, 1998)
Registered by the IGU Technical Secretariat No. 2907
| State name | |
| Republic of Belarus | |
| The Republic of Kazakhstan | |
| Republic of Kyrgyzstan | Kyrgyzstandard |
| The Republic of Moldova | Moldovastandart |
| Russian Federation | Gosstandart of Russia |
| The Republic of Tajikistan | Tajikstandart |
| Turkmenistan | |
| The Republic of Uzbekistan | Uzgosstandart |
| State Standard of Ukraine |
Amendment No. 10 was adopted by the Interstate Council for Standardization, Metrology and Certification (Minutes No. 17 of June 22, 2000)
Registered by the IGU Technical Secretariat No. 3526
| State name | Name of the national standardization body |
| The Republic of Azerbaijan | Azgosstandart |
| Republic of Belarus | State Standard of the Republic of Belarus |
| Gruzstandart |
|
| The Republic of Kazakhstan | Gosstandart of the Republic of Kazakhstan |
| Republic of Kyrgyzstan | Kyrgyzstandard |
| The Republic of Moldova | Moldovastandart |
| Russian Federation | Gosstandart of Russia |
| The Republic of Tajikistan | Tajikstandart |
| Turkmenistan | Glavgosluzhba "Turkmenstandartlary" |
3. REPLACE GOST 2.107-68, GOST 2.109-68, GOST 5292-60 in part of Sec. VIII
4. REFERENCE REFERENCE DOCUMENTS
(Modified edition, Amendment No. 11)
5. EDITION (June 2002) with Amendments No. 1, 2, 3.4, 5, 6, 7, 8, 9, 10, approved in February 1980, November 1981, May 1984, December 1984 March 1985, September 1985, March 1986, September 1987, February 1999, December 2000 (IMS No. 4-80, 4-82, 8-84, 3-85, 5-85,12-85, 6-86, 12-87, 5-99, 3-2001)
The flat shape obtained by aligning with the plane of all its faces is called the unfolding of the surface of a polyhedron. The unfolding of faceted surfaces is performed for cutting sheet material in the manufacture of parts or for determining the surface area of parts covered with various materials. Determination of the area is important for various coatings, carried out both for decorative purposes and in order to impart certain properties to the surface, for example, increased electrical conductivity, as well as with various chemical surface treatment methods.
To construct a flat pattern of a faceted surface, it is necessary to determine the dimensions of its faces. Note that the construction of any face of a polyhedron can be performed by dividing it into triangles. The length of the sides of the triangle, in turn, can be determined by any of the known methods.
Unfold the surface of the pyramid. The construction of a sweep of the side surface of the pyramid can be carried out in the following sequence:
determine the length of the ribs and sides of the base of the pyramid; carry out a drawing of a sweep by sequential construction of triangles - the faces of the pyramid.
An example of building a flat surface of a triangular pyramid SABC is shown in Figures 6.14 and 6.15. For the convenience of construction in Figure 6.14, the side edges of the pyramid are extended to the intersection with the plane N. This made it possible to determine the length of the segments on the horizontal projection 1-2, 2-3, 3-4 new base of the pyramid. Side rib length S-l, S-2, S-3 found by rotating them around the vertical axis - segments s "1 1", s "2 1", s "3 1". Segments were found on them s "a 1", s "b 1", s "c 1". According to the found segments in Figure 6.15, a side surface unfolding is built Solo2o3o1o and then S 0 A 0 BoCoAo. On the segment A 0 C 0 the actual size of the triangle is built A 0 B 0 C 0 on the sides of A 0 B 0 and C0B0, by the found method of a right-angled triangle (see Fig. 2.9).
Creation of a flat pattern of a prismatic surface can be produced in several ways - normal section, triangles.
With the normal section method, it is advisable to construct a sweep of a prismatic surface in the following order (Fig. 6.16):
intersect a prismatic surface with a construction plane perpendicular to its edges (P is perpendicular to 1-2;normal section);
unfold the constructed polyline (А0В0С0D0) the intersection of the auxiliary plane with the prismatic surface, having determined the length of its segments (A0B0, B 0 C 0, C 0 D 0);
on perpendiculars to the expanded line of intersection (A0D0), set the length of the edge segments of the prismatic surface (A 0 2 0, BoZo, Bo4o, Co5o, Co6o, Do7o, Do8o)and connect their ends with straight line segments.
An example of constructing a sweep of the lateral surface of an inclined prism in the drawing is shown in Figures 6.17 and 6.18. To build an auxiliary plane P, perpendicular to the prism edges, an additional projection plane is selected T, parallel to the edges of the prism and perpendicular to the plane N. Auxiliary plane P is given by the trace P t on the projection plane T S (pl. S perpendicular to T).
According to the method of triangles, the development of a prismatic surface is as follows: quadrangles (faces) are divided by diagonals into triangles; determine the lengths of the sides of the triangles; perform a sweep drawing by sequentially building triangles into which the faces are divided.
Benzene is an unsaturated compound, but we found out that there are no double bonds in its structure, but there is an aromatic bond - a delocalized electron cloud. Typical reactions of unsaturated hydrocarbons - electrophilic addition and oxidation - are not typical for benzene. So, it does not discolor bromine water, does not give the Wagner reaction (oxidation with a solution of potassium permanganate at room temperature). For benzene, reactions are characteristic that do not lead to a violation of the closed conjugated system - substitution reactions. To find out what type of substitution (radical, electrophilic, nucleophilic) is characteristic of benzene, remember its electronic structure: the σ-skeleton of the molecule is flat, and an aromatic cloud is located above and below the plane. To interact with this aromatic cloud, the reagent must be electrophilic. So, benzene (and aromatic compounds in general) is characterized by electrophilic substitution reactions ... Examples of S E reactions are:
At the first stage, the electrophile approaches the benzene molecule and interacts with the entire aromatic cloud (they are attracted to each other). Formed π-complex... A pair of electrons is needed to form a new carbon-electrophile covalent bond. Electrophile pulls it out of the aromatic cloud, it is formed σ-complex... It is not a closed coupled system, since the carbon atom, which formed a new σ-bond, passed into sp 3 -hybridization (it left the plane and no longer has a non-hybrid p z-orbital). The remaining five carbon atoms continue to participate in conjugation, forming a common electron cloud, in which four electrons are delocalized (6-2 = 4), therefore the positive charge in the σ-complex is indicated not on a specific carbon atom, but in the center of an open ring. So the σ-complex is not an aromatic structure. In order to restore aromaticity, it needs to split off a hydrogen proton (H +). It is taken by the nucleophile (Nu -) remaining in the reaction medium. Two electrons of the C-H bond return to the aromatic cloud, the carbon atom again becomes
sp 2 -hybridized and can participate in conjugation.
The limiting stage of the electrophilic substitution reaction is the stage of formation of the σ-complex, since in this case, a loss of aromaticity occurs, which requires energy consumption.
Various reactions of electrophilic substitution in benzene proceed according to a general mechanism and differ only in the stage of formation of the electrophilic particle.
Nitration reaction benzene proceeds under the action of a mixture of concentrated nitric and sulfuric acids (see the reaction scheme above). Let's consider its mechanism.
 |
In the first stage of the reaction, nitric acid reacts with sulfuric. In this case, nitric acid acts as a base, accepting a proton from a sulfuric acid molecule (according to Bronsted's theory, an acid is a molecule or ion that donates a proton, and a base is a molecule or ion that accepts a proton of hydrogen). A protonated nitric acid is formed, which, by splitting off a water molecule, turns into a nitronium cation, or nitronium cation. This is an electrophilic particle. Thus, sulfuric acid acts as a catalyst, taking part in the formation of an electrophilic reagent. The second role of sulfuric acid is that of a dehydrating agent. Water must be diverted from the reaction sphere in order to shift its equilibrium to the right.
After the formation of an electrophile - nitronium cation - the reaction proceeds according to the general mechanism, through the formation of π- and
σ-complexes:
Please note: at the stage of transformation of the σ-complex into nitrobenzene (the stage of returning aromaticity), the hydrogen proton is split off by the action of the sulfuric acid anion, while sulfuric acid is again formed, which proves that it was the catalyst for this reaction.
 Catalyst halogenation reactions are the so-called Lewis acids (according to the Lewis theory, acids are neutral molecules or ions capable of accepting a pair of electrons): FeCl 3, FeBr 3, AlCl 3, AlBr 3, etc. A catalyst is needed to polarize the halogen molecule. The Lewis acid displaces the lone electron pair of chlorine onto itself, forming a complex in which a partial positive charge is concentrated on one of the chlorine atoms:
Catalyst halogenation reactions are the so-called Lewis acids (according to the Lewis theory, acids are neutral molecules or ions capable of accepting a pair of electrons): FeCl 3, FeBr 3, AlCl 3, AlBr 3, etc. A catalyst is needed to polarize the halogen molecule. The Lewis acid displaces the lone electron pair of chlorine onto itself, forming a complex in which a partial positive charge is concentrated on one of the chlorine atoms:
At the stage of π-complex formation, further polarization of the Cl-Cl bond occurs, and it is broken heterolytically, with Cl + immediately participating in the formation of the σ-complex.
 Similarly proceed alkylation reactions(Friedel-Crafts reaction).
Similarly proceed alkylation reactions(Friedel-Crafts reaction).
 |
The C-Cl bond in methyl chloride is not polar enough to break heterolytically. Under the action of the Lewis acid, the partial positive charge on the carbon atom increases, and the complex of the reagent with the catalyst is a stronger electrophile than the starting methyl chloride.
Sulfonation reaction benzene proceeds under the action of oleum (solution of sulfuric anhydride SO 3 in concentrated sulfuric acid).
The sulfuric anhydride molecule is electrophile due to the large partial positive charge on the sulfur atom.
 |
When a π-complex is formed, the S = O bond (primarily a π-bond) is polarized and broken in a heterolytic manner, therefore, when a σ-complex is formed on the oxygen atom, a complete negative charge arises. To restore aromaticity, a hydrogen proton is split off from the ring carbon atom and passes to negatively charged oxygen. Benzenesulfonic acid is formed.
When we consider the reactions of electrophilic substitution in benzene, we are not faced with the question of the position in which the reaction proceeds, since all carbon atoms are absolutely equal. It is another matter if there is already a substituent in the benzene ring. In this case, as a result of electrophilic substitution, the formation of three isomers is in principle possible:
 |
To answer the question of which of these possible products is predominant, it is necessary to consider the electronic effects of the substituent.
Let us digress from the reactions of electrophilic substitution in benzene and its derivatives and consider electronic effects in general.
Mutual influence of atoms in organic molecules
connections. Electronic effects
Atoms and atomic groups in the molecules of organic compounds affect each other, and not only atoms directly bonded to each other. This influence is somehow transmitted through the molecule. The transfer of the influence of atoms in molecules due to the polarization of bonds is called electronic effects ... There are two types of electronic effects: inductive and mesomeric effects.
Inductive effect- this is the transfer of the influence of substituents along the chain of σ-bonds due to their polarization. The inductive effect is denoted by the symbol I. Let's consider it using the example of 1-chlorobutane:
The C-Cl bond is polar due to the higher electronegativity of chlorine. A partial positive charge (δ +) arises on the carbon atom. The electron pair of the next σ-bond is shifted towards the electron-deficient carbon atom, i.e. polarized. Due to this, a partial positive charge (δ + ') also arises on the next carbon atom, etc. So chlorine induces polarization not only of the "intrinsic" σ-bond, but also of the subsequent ones in the chain. Note that each subsequent partial positive charge is less than the previous one (δ +> δ + ’> δ +’ ’> δ +’ ’’), i.e. the inductive effect is transmitted along the circuit with damping. This can be explained by the low polarizability of σ-bonds. It is generally accepted that the inductive effect extends to 3-4 σ-bonds. In the given example, the chlorine atom shifts the electron density along the bond chain to myself... This effect is called negative inductive effect and is referred to as –I Cl.
Most of the substituents exhibit a negative inductive effect, because their structure contains atoms that are more electronegative than hydrogen (the inductive effect of hydrogen is assumed to be zero). For example: -F, -Cl, -Br, -I, -OH, -NH 2, -NO 2,
-COOH,> C = O.
 |  |
||
If the substituent shifts the electron density along the chain of σ-bonds Push, it has a positive inductive effect (+ I). For example:

Oxygen with a total negative charge has a positive inductive effect.
![]()
In the propene molecule, the carbon of the methyl group is sp 3 -hybridized, and the carbon atoms at the sp 2 double bond are hybridized, i.e. more electronegative. Therefore, the methyl group shifts the electron density away from itself, exhibiting a positive inductive effect (+ I CH 3).
So, the inductive effect can manifest itself in any molecule in which there are atoms of different electronegativity.
Mesomeric effect Is the transfer of the electronic influence of substituents in conjugated systems through the polarization of π-bonds. The mesomeric effect is transmitted without attenuation, because π-bonds are easily polarized. Please note: only those substituents that are themselves part of the conjugated system have a mesomeric effect. For example:
The mesomeric effect can be either positive (+ M) or negative (-M).
In the vinyl chloride molecule, the lone electron pair of chlorine participates in p, π-conjugation, i.e. the contribution of chlorine to the conjugated system is greater than that of each of the carbon atoms. Therefore, chlorine exhibits a positive mesomeric effect.
 An acrylic aldehyde molecule is
An acrylic aldehyde molecule is
π.π-conjugate system. The oxygen atom gives up one electron to conjugation - the same as each carbon atom, but the electronegativity of oxygen is higher than that of carbon, therefore oxygen shifts the electron density of the conjugated system towards itself, the aldehyde group as a whole exhibits a negative mesomeric effect.
So, substituents that donate two electrons to conjugation have a positive mesomeric effect. These include:
a) substituents with a complete negative charge, for example, –O -;
b) substituents, in the structure of which there are atoms with lone electron pairs on the p z -orbital, for example: -NH 2, -OH,
-F, -Cl, -Br-, -I, -OR (-OCH 3, -OC 2 H 5).
The substituents shifting the electron density along the conjugated system towards themselves exhibit a negative mesomeric effect. These include substituents in the structure of which there are double bonds, for example:
 |
The substituent can exhibit both inductive and mesomeric effects at the same time. In some cases, the direction of these effects is the same (for example, -I and -M), in others - they act in opposite directions (for example, -I and + M). How, in these cases, can one determine the general effect of a substituent on the rest of the molecule (in other words, how to determine whether a given substituent is electron-donating or electron-withdrawing)? Substituents that increase the electron density in the rest of the molecule are called electron donor, and substituents that lower the electron density in the rest of the molecule are called electron acceptor.
To determine the overall effect of a substituent, it is necessary to compare its electronic effects in magnitude. If the positive effect prevails, the substituent is electron donor. If the negative effect prevails, the substituent is electron-withdrawing. It should be noted that, as a rule, the mesomeric effect is more pronounced than the inductive one (due to the greater ability of π-bonds to polarize). However, there are exceptions to this rule: the inductive effect of halogens is more pronounced than the mesomeric one.
Let's consider specific examples:
In this compound, the amino group is an electron-donating substituent, since its positive mesomeric effect is more pronounced than the negative inductive one.
 In this compound, the amino group is an electron-withdrawing spotter, since exhibits only a negative inductive effect.
In this compound, the amino group is an electron-withdrawing spotter, since exhibits only a negative inductive effect.
In the phenol molecule, the hydroxyl group is an electron-donor substituent due to the predominance of the positive mesomeric effect over the negative inductive one.
 In the benzyl alcohol molecule, the hydroxyl group does not participate in conjugation and exhibits only a negative inductive effect. Therefore, it is an electron-withdrawing substituent.
In the benzyl alcohol molecule, the hydroxyl group does not participate in conjugation and exhibits only a negative inductive effect. Therefore, it is an electron-withdrawing substituent.
These examples show that it is impossible to consider the influence of any substituent in general, but it is necessary to consider its influence in a specific molecule.
 Only halogens are always electron-withdrawing substituents, since their negative inductive effect is more pronounced than the positive mesomeric effect. For example:
Only halogens are always electron-withdrawing substituents, since their negative inductive effect is more pronounced than the positive mesomeric effect. For example:

Now let's get back to electrophilic substitution reactions in benzene derivatives. So, we found out that the substituent already present in the ring affects the course of electrophilic substitution reactions. How is this influence expressed?
The substituent affects the reaction rate S E and the position of the second substituent introduced into the ring... Let's consider both of these aspects of influence.
Influence on reaction rate... The higher the electron density in the ring, the easier the reactions of electrophilic substitution are. It is clear that electron-donating substituents facilitate the S E reactions (they are cycle activators), and electron-withdrawing substituents make them more difficult (deactivate the cycle). Therefore, electrophilic substitution reactions in benzene derivatives containing electron-withdrawing substituents are carried out under more severe conditions.
 Let us compare the activity of phenol, toluene, benzene, chlorobenzene and nitrobenzene in the nitration reaction.
Let us compare the activity of phenol, toluene, benzene, chlorobenzene and nitrobenzene in the nitration reaction.
Since phenol and toluene contain electron-donating substituents, they are more active in S E reactions than benzene. On the contrary, chlorobenzene and nitrobenzene are less active in these reactions than benzene, because contain electron-withdrawing substituents. Phenol is more active than toluene due to the positive mesomeric effect of the OH group. Chlorine is not as strong an electron-withdrawing substituent as the nitro group, because the nitro group exhibits both negative inductive and negative mesomeric effects. So, in this series, the activity in electrophilic substitution reactions decreases from phenol to nitrobenzene. It has been experimentally established that if the rate of the benzene nitration reaction is taken as 1, then this series will look like this:
The second aspect of the influence of a substituent in an aromatic ring on the course of electrophilic substitution reactions is the so-called orienting action of substitutes... All substituents can be subdivided into two groups: ortho-, para-orientants (substituents of the 1st kind) and meta-orientants (substituents of the 2nd kind).
TO substitutes of the 1st kind include: -OH, -O -, -NH 2, alkyl groups (-CH 3, -C 2 H 5, etc.) and halogens. You can see that all of these substituents exhibit a positive inductive effect and / or a positive mesomeric effect. All of them, except for halogens, increase the electron density in the ring, especially in the ortho and para positions. Therefore, the electrophile is directed to these positions. Let's take a look at phenol as an example:
Due to the positive mesomeric effect of the hydroxyl group, the electron density is redistributed over the conjugated system, and it is especially increased in the ortho and para positions.
 When phenol is brominated, a mixture of ortho- and para-bromophenol is formed:
When phenol is brominated, a mixture of ortho- and para-bromophenol is formed:
If bromination is carried out in a polar solvent (bromine water) and an excess of bromine is used, the reaction proceeds at once in three positions:
 |
Substitutes of the 2nd kind are: -NH 3 +, -COOH, -CHO (aldehyde group), -NO 2, -SO 3 H. All these substituents lower the electron density in the aromatic ring, but because of its redistribution in the meta-positions, it is not lowered so strong as in ortho and para. Let's consider this using benzoic acid as an example:
The carboxyl group exhibits negative inductive and negative mesomeric effects. Due to the redistribution over the conjugated system in the meta-positions, the electron density remains higher than in the ortho- and para-, so the electrophile will attack the meta-positions:
 |
The first group of reactions is substitution reactions. We said that arenes do not have multiple bonds in the structure of the molecule, but contain a conjugated system of six electrons, which is very stable and gives additional strength to the benzene ring. Therefore, in chemical reactions, first of all, the replacement of hydrogen atoms occurs, and not the destruction of the benzene ring.
We have already encountered substitution reactions when talking about alkanes, but for them these reactions proceeded according to a radical mechanism, while arenes are characterized by the ionic mechanism of substitution reactions.
First chemical property - halogenation. Substitution of a hydrogen atom for a halogen atom - chlorine or bromine.
The reaction proceeds with heating and always with the participation of a catalyst. In the case of chlorine, this can be aluminum chloride or ferric chloride three. The catalyst polarizes the halogen molecule, resulting in heterolytic bond cleavage and ions.
A positively charged chlorine ion and reacts with benzene.
If the reaction occurs with bromine, then the catalyst is ferric bromide or aluminum bromide.

It is important to note that the reaction occurs with molecular bromine and not with bromine water. Benzene does not react with bromine water.
Halogenation of benzene homologues has its own characteristics. In the toluene molecule, the methyl group facilitates the substitution in the ring, the reactivity increases, and the reaction proceeds under milder conditions, that is, already at room temperature.

It is important to note that the substitution always occurs at the ortho and para positions, so a mixture of isomers is obtained.
Second property - nitration of benzene, introduction of a nitro group into the benzene ring.

A heavy yellowish liquid with the smell of bitter almonds is formed - nitrobenzene, so the reaction can be of high quality to benzene. For nitration, a nitrating mixture of concentrated nitric and sulfuric acids is used. The reaction is carried out with heating.
Let me remind you that for the nitration of alkanes in the Konovalov reaction, we used dilute nitric acid without the addition of sulfuric acid.
During the nitration of toluene, as well as during halogenation, a mixture of ortho and para isomers is formed.
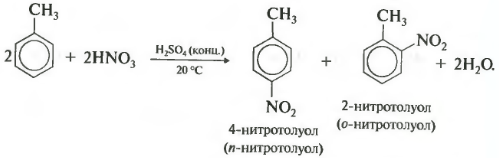
Third property - alkylation of benzene with haloalkanes.

This reaction allows the introduction of a hydrocarbon radical into the benzene ring and can be considered a way to obtain benzene homologues. Aluminum chloride is used as a catalyst, which promotes the decomposition of the haloalkane molecule into ions. Heating is also necessary.
Fourth property - alkylation of benzene with alkenes.

In this way, you can get, for example, cumene or ethylbenzene. The catalyst is aluminum chloride.
2. Reactions of addition to benzene
The second group of reactions is addition reactions. We said that these reactions are not typical, but they are possible under rather harsh conditions with the destruction of the pi-electron cloud and the formation of six sigma bonds.
Fifth property in the general list - hydrogenation, addition of hydrogen.
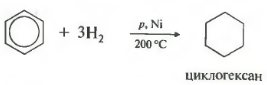
Temperature, pressure, catalyst nickel or platinum. Toluene can react in the same way.
Sixth property - chlorination. Please note that we are talking specifically about the interaction with chlorine, since bromine does not enter into this reaction.
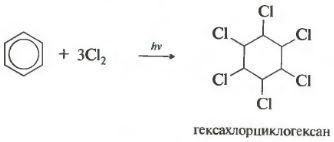
This reaction takes place under severe ultraviolet radiation. Formed hexachlorocyclohexane, another name is hexachlorane, a solid.
It is important to remember that for benzene not possible reactions of addition of hydrogen halides (hydrohalogenation) and addition of water (hydration).
3. Substitution in the side chain of benzene homologues
The third group of reactions concerns only benzene homologues - this is the substitution in the side chain.
Seventh property in the general list - halogenation at the alpha carbon atom in the side chain.

The reaction occurs when heated or irradiated and always only on alpha carbon. As halogenation continues, the second halogen atom will return to the alpha position.
4. Oxidation of benzene homologues
The fourth group of reactions is oxidation.
The benzene ring is too strong, so benzene does not oxidize potassium permanganate - does not discolor its solution. This is very important to remember.
On the other hand, benzene homologues are oxidized by an acidified solution of potassium permanganate when heated. And this is the eighth chemical property.

It turns out benzoic acid. Discoloration of the solution is observed. In this case, no matter how long the carbon chain of the substituent is, it always breaks after the first carbon atom and the alpha atom is oxidized to the carboxyl group with the formation of benzoic acid. The rest of the molecule is oxidized to the corresponding acid or, if it is only one carbon atom, to carbon dioxide.
If the benzene homologue has more than one hydrocarbon substituent on the aromatic ring, then the oxidation proceeds according to the same rules - the carbon in the alpha position is oxidized.
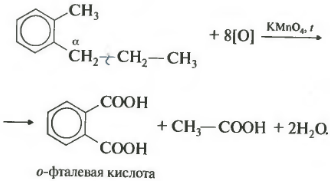
This example produces a dibasic aromatic acid called phthalic acid.
In a special way I will note the oxidation of cumene, isopropylbenzene, by atmospheric oxygen in the presence of sulfuric acid.

This is the so-called cumene method for producing phenol. As a rule, one has to deal with this reaction in matters relating to the production of phenol. This is an industrial way.
Ninth property - combustion, complete oxidation with oxygen. Benzene and its homologues burn to carbon dioxide and water.
Let's write the equation of benzene combustion in general form.
According to the law of conservation of mass, there should be as many atoms on the left as there are atoms on the right. Because in chemical reactions atoms do not disappear, but the order of bonds between them simply changes. So there will be as many carbon dioxide molecules as there are carbon atoms in the arene molecule, since the molecule contains one carbon atom. That is n CO 2 molecules. There will be two times less water molecules than hydrogen atoms, that is, (2n-6) / 2, which means n-3.
The oxygen atoms on the left and right are the same number. On the right, there are 2n carbon dioxide, because each molecule has two oxygen atoms, plus n-3 from water, for a total of 3n-3. On the left, there are the same number of oxygen atoms - 3n-3, which means there are half the number of molecules, because the molecule contains two atoms. That is (3n-3) / 2 oxygen molecules.
Thus, we have compiled the combustion equation for benzene homologues in general form.
The cyclic structure of benzene was first proposed by F.A. Kekule in 1865

Friedrich August Kekule von Stradonitz was an outstanding German chemist of the 19th century. In 1854 he discovered the first organic compound containing sulfur - thioacetic acid (thioethanic acid). In addition, he established the structure of diazo compounds. However, his most famous contribution to the development of chemistry is the establishment of the structure of benzene (1866). Kekule showed that the double bonds of benzene alternate around the ring (this idea first occurred to him in a dream). He later showed that the two possible double bond arrangements are identical and that the benzene ring is a hybrid between the two. Thus, he anticipated the concept of resonance (mesomerism), which appeared in the theory of chemical bonding in the early 1930s.
If benzene really had such a structure, then its 1,2-disubstituted derivatives should have two isomers. For example,

However, none of the 1,2-disubstituted benzenes can isolate two isomers.
Therefore, later Kekulé suggested that the benzene molecule exists as two structures rapidly passing into each other:

Note that such schematic representations of benzene molecules and their derivatives usually do not indicate the hydrogen atoms attached to the carbon atoms of the benzene ring.
In modern chemistry, a benzene molecule is considered as a resonant hybrid of these two limiting resonance forms (see Section 2.1). Another description of the benzene molecule is based on the consideration of its molecular orbitals. In sect. 3.1, it was indicated that the -electrons located in the -bonding orbitals are delocalized between all the carbon atoms of the benzene ring and form an -electron cloud. In accordance with this concept, the benzene molecule can be conventionally depicted as follows:
Experimental data confirm the presence of just such a structure in benzene. If benzene had the structure that Kekulé initially suggested, with three conjugated double bonds, then benzene should have entered into addition reactions like alkenes. However, as mentioned above, benzene does not undergo addition reactions. In addition, benzene is more stable than if it had three isolated double bonds. In sect. 5.3 it was indicated that the enthalpy of hydrogenation of benzene with the formation of cyclohexane has a greater negative
Table 18.3. Length of various carbon-carbon bonds


Rice. 18.6. The geometric structure of the benzene molecule.
value than three times the hydrogenation enthalpy of cyclohexene. The difference between these quantities is usually called the enthalpy of delocalization, resonance energy, or the stabilization energy of benzene.
All carbon-carbon bonds in the benzene ring have the same length, which is less than the length of C-C bonds in alkanes, but longer than the length of C = C bonds in alkenes (Table 18.3). This confirms that the carbon-carbon bonds in benzene are a hybrid between single and double bonds.
The benzene molecule has a flat structure, which is shown in Fig. 18.6.
Physical properties
Benzene under normal conditions is a colorless liquid that freezes at 5.5 ° C and boils at 80 ° C. It has a characteristic pleasant smell, but, as mentioned above, is highly toxic. Benzene is immiscible with water, and in the benzene system, water forms the upper of two layers. However, it dissolves in non-polar organic solvents and is itself a good solvent for other organic compounds.
Chemical properties
Although benzene undergoes certain addition reactions (see below), it does not exhibit the reactivity typical of alkenes. For example, it does not discolor bromine water or β-ion solution. In addition, benzene does not
enters into addition reactions with strong acids, for example, with hydrochloric or sulfuric acid.
At the same time, benzene takes part in a number of electrophilic substitution reactions. The products of reactions of this type are aromatic compounds, since the delocalized β-electronic system of benzene is retained in these reactions. The general mechanism of the replacement of a hydrogen atom on a benzene ring by an electrophile is described in Sec. 17.3. Examples of electrophilic substitution of benzene are nitration, halogenation, sulfonation, and Friedel-Crafts reactions.
Nitration. Benzene can be nitrated (introduce a group into it) by treating it with a mixture of concentrated nitric and sulfuric acids:

Nitrobenzene
The conditions for this reaction and its mechanism are described in Sec. 17.3.
Nitrobenzene is a pale yellow liquid with a characteristic almond odor. When nitrating benzene, in addition to nitrobenzene, crystals of 1,3-dinitrobenzene are also formed, which is the product of the following reaction:

Halogenation. If benzene is mixed in the dark with chlorine or bromine, no reaction will occur. However, in the presence of catalysts with Lewis acid properties, electrophilic substitution reactions occur in such mixtures. Typical catalysts for these reactions are iron (III) bromide and aluminum chloride. The effect of these catalysts is that they create polarization in the halogen molecules, which then form a complex with the catalyst:
although there is no direct evidence that free ions are formed. The mechanism of benzene bromination with iron (III) bromide as an ion carrier can be represented as follows:

Sulfonation. Benzene can be sulfonated (to replace a hydrogen atom with a sulfo group) by refluxing its mixture with concentrated sulfuric acid for several hours. Instead, benzene can be gently heated in a mixture with fuming sulfuric acid. Fuming sulfuric acid contains sulfur trioxide. The mechanism of this reaction can be represented by the diagram

Friedel-Crafts reactions. Friedel-Crafts reactions were originally called condensation reactions between aromatic compounds and alkyl halides in the presence of anhydrous aluminum chloride catalyst.
In condensation reactions, two molecules of reagents (or one reagent) combine with each other, forming a molecule of a new compound, while a molecule of some simple compound, such as water or hydrogen chloride, is split off (eliminated) from them.
Currently, the Friedel-Crafts reaction is called any electrophilic substitution of an aromatic compound, in which the role of the electrophile is played by any carbocation or a strongly polarized complex with a positively charged carbon atom. The electrophilic agent is usually an alkyl halide or a chloride of some carboxylic acid, although it can also be, for example, an alkene or an alcohol. Anhydrous aluminum chloride is commonly used as a catalyst for these reactions. Friedel-Crafts reactions are usually divided into two types: alkylation and acylation.
Alkylation. In Friedel-Crafts reactions of this type, one or more hydrogen atoms in the benzene ring are replaced by alkyl groups. For example, when a mixture of benzene and chloromethane is gently heated in the presence of anhydrous aluminum chloride, methylbenzene is formed. Chloromethane plays the role of an electrophilic agent in this reaction. It is polarized by aluminum chloride in the same way as it happens with halogen molecules:
The mechanism of the considered reaction can be represented as follows:

It should be noted that in this condensation reaction between benzene and chloromethane, the hydrogen chloride molecule is eliminated. Note also that the real existence of the methyl carbocation in the form of a free ion is doubtful.
Alkylation of benzene with chloromethane in the presence of anhydrous aluminum chloride catalyst does not end with the formation of methylbenzene. In this reaction, further alkylation of the benzene ring occurs, leading to the formation of 1,2-dimethylbenzene:

Acylation. In Friedel-Crafts reactions of this type, the hydrogen atom in the benzene ring is replaced by an acyl group, resulting in the formation of an aromatic ketone.
The acyl group has the general formula
The systematic name of an acyl compound is formed by replacing the suffix and ending -ova in the name of the corresponding carboxylic acid, of which this acyl compound is a derivative, with the suffix - (o) yl. For example

The acylation of benzene is carried out using a chloride or anhydride of a carboxylic acid in the presence of anhydrous aluminum chloride catalyst. For example

This reaction is a condensation in which the hydrogen chloride molecule is cleaved off. Note also that the name "phenyl" is often used to refer to the benzene ring in compounds where benzene is not the main group:

Addition reactions. Although benzene is most characterized by electrophilic substitution reactions, it also undergoes some addition reactions. We have already met one of them. This is the hydrogenation of benzene (see Section 5.3). When a mixture of benzene with hydrogen is passed over the surface of a finely ground nickel catalyst at a temperature of 150-160 ° C, a whole sequence of reactions occurs, which ends with the formation of cyclohexane. The overall stoichiometric equation for this reaction can be represented as follows:

Under the influence of ultraviolet radiation or direct sunlight, benzene also enters into an addition reaction with chlorine. This reaction is carried out by a complex radical mechanism. Its final product is 1,2,3,4,5,6-hexachlorocyclohexane:

A similar reaction occurs between benzene and bromine when exposed to ultraviolet radiation or sunlight.
Oxidation. Benzene and benzene ring in other aromatic compounds, generally speaking, are resistant to oxidation even with such strong oxidizing agents as acidic or alkaline potassium permanganate solution. However, benzene and other aromatics burn in air or oxygen to form a very smoky flame, which is typical of hydrocarbons with a high relative carbon content.
DEFINITION
Benzene is a colorless liquid with a characteristic odor; boiling point 80.1 o C, melting point 5.5 o C. Insoluble in water, toxic.
The aromatic properties of benzene, determined by the peculiarities of its structure, are expressed in the relative stability of the benzene nucleus, despite the lack of composition of benzene. So, unlike unsaturated compounds with ethylene double bonds, benzene is resistant to oxidants.
Rice. 1. The structure of the benzene molecule according to Kekule.
Getting benzene
The main methods for producing benzene include:
- dehydrocyclization of hexane (catalysts - Pt, Cr 3 O 2)
CH 3 - (CH 2) 4 -CH 3 → C 6 H 6 + 4H 2 (t o C, p, kat = Cr 2 O 3);
- dehydrogenation of cyclohexane
C 6 H 12 → C 6 H 6 + 3H 2 (t o C, kat = Pt, Ni);
- trimerization of acetylene (the reaction proceeds when heated to 600 o С, the catalyst is activated carbon)
3HC≡CH → C 6 H 6 (t = 600 o C, kat = C activ).
Chemical properties of benzene
For benzene, substitution reactions occurring by an electrophilic mechanism are characteristic:
Halogenation (benzene interacts with chlorine and bromine in the presence of catalysts - anhydrous AlCl 3, FeCl 3, AlBr 3)
C 6 H 6 + Cl 2 = C 6 H 5 -Cl + HCl;
- nitration (benzene easily reacts with a nitrating mixture - a mixture of concentrated nitric and sulfuric acids)

- alkylation with alkenes
C 6 H 6 + CH 2 = CH-CH 3 → C 6 H 5 -CH (CH 3) 2
The addition reactions to benzene lead to the destruction of the aromatic system and proceed only under severe conditions:
- hydrogenation (reaction product - cyclohexane)
C 6 H 6 + 3H 2 → C 6 H 12 (t o C, kat = Pt);
- addition of chlorine (proceeds under the action of UV radiation with the formation of a solid product - hexachlorocyclohexane (hexachlorane) - C 6 H 6 Cl 6)
C 6 H 6 + 6Cl 2 → C 6 H 6 Cl 6.
The use of benzene
Benzene is widely used in industrial organic chemistry. Almost all compounds containing benzene rings are obtained from benzene, for example, styrene, phenol, aniline, halogenated arenes. Benzene is used for the synthesis of dyes, surfactants, pharmaceuticals.
Examples of problem solving
EXAMPLE 1
| Exercise | The vapor density of the substance is 3.482 g / l. Its pyrolysis gave 6 g of soot and 5.6 L of hydrogen. Determine the formula for this substance. |
| Solution | Soot is carbon. Let us find the amount of soot substance based on the conditions of the problem (the molar mass of carbon is 12 g / mol): n (C) = m (C) / M (C); n (C) = 6/12 = 0.5 mol. Let's calculate the amount of hydrogen substance: n (H 2) = V (H 2) / V m; n (H 2) = 5.6 / 22.4 = 0.25 mol. This means that the amount of substance of one hydrogen atom will be equal to: n (H) = 2 × 0.25 = 0.5 mol. Let's designate the number of carbon atoms in a hydrocarbon molecule as "x", and the number of hydrogen atoms as "y", then the ratio of these atoms in the molecule: x: y = 0.5: 0.5 = 1: 1. Then the simplest hydrocarbon formula will be expressed by the CH composition. The molecular weight of a molecule of composition CH is equal to: M (CH) = 13 g / mol Let us find the molecular weight of the hydrocarbon based on the conditions of the problem: M (C x H y) = ρ × V m; M (C x H y) = 3.482 × 22.4 = 78 g / mol. Let's define the true formula of the hydrocarbon: k = M (C x H y) / M (CH) = 78/13 = 6, therefore, the coefficients "x" and "y" must be multiplied by 6 and then the hydrocarbon formula will take the form C 6 H 6. This is benzene. |
| Answer | The desired hydrocarbon has the composition C 6 H 6. This is benzene. |
EXAMPLE 2
| Exercise | Calculate the amount of acetylene that will be needed to obtain 400 ml of benzene (density 0.8 g / ml). |
| Solution | Let us write the equation for the reaction of obtaining benzene from acetylene: |








